LSM 510 NLO and LSM 510 META NLO Multiphoton Laser Scanning Microscopes Deep Insights Carefully Gained
|
|
|
- Georgia Chambers
- 6 years ago
- Views:
Transcription
1 Microscopy from Carl Zeiss LSM 510 NLO and LSM 510 META NLO Multiphoton Laser Scanning Microscopes Deep Insights Carefully Gained
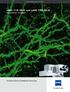
2 LSM 510 NLO and LSM 510 META NLO Deep Insights Carefully Gained In multiphoton microscopy, femtosecond lasers make it possible to create and detect fluorescence signals up to 500 µm deep within tissue. At the same time, the excitation of the fluorochrome by NIR radiation, which is limited to the focal spot, allows careful examination of living cells and tissue specimens. As many conventional fluorochromes used in multiphoton microscopy show very wide excitation spectra, a single wavelength can simultaneously excite a whole variety of these dyes in a specimen. The system solutions offered by Carl Zeiss are superior in efficiency, sensitivity and flexibility. Bristle-worm (Brania), labeled for tubulin (FITC, green) and serotonin (CY3, red). Both fluorochromes were excited simultaneously at 780 nm with a femtosecond laser. Specimen: Dr. R. Hessling, University of Osnabrück, Germany
3 Contents The Principle of Multiphoton Microscopy 4 System Components 6 Femtosecond Laser and NDD 8 Signal Processor and Laser Attenuation 10 Auto Z Brightness Correction 11 Region of Interest (ROI) 12 Spot Scan and Spot Bleach 13 Z Bleach 14 Visible Light and Near Infrared 16 META Detection 17 Excitation Fingerprinting 18 Specification 20 System Overview 24
4 2
5 3
6 The Principle of Multiphoton Microscopy Excitation on the Spot So far researchers attempting to generate fluorescence signals deep inside fluorescent tissue sections quickly came up against limitations. This was caused by the increase in absorption and scattering of the exciting and emitted light when focusing deeper into the tissue. The LSM 510 NLO and LSM 510 META NLO conquer this drawback: they can image fluorescence signals emitted at depths of several hundreds of µm. This is mainly due to two properties: The bigger longer wavelength of the femtosecond laser used, and the restriction of fluorescence excitation to the focal spot. GFP-expressing astrocytes, about 150 µm deep inside a fixed mouse brain section. Excitation at 488 nm. Heavy scattering of the fluorescence generated outside the focus prevents distinct cell imaging. Energy Energy diagram of fluorescence generation with single photon excitation. Femtosecond lasers excite the dye only in the focal spot. Detector Pinhole in the confocal plane 4 Prof. W. Denk, Max Planck Institute of Medical Research, Heidelberg, Germany To get high-resolution images from the depths of light-scattering tissue, there is no alternative to two-photon microscopy. Half a millimeter below the surface of a brain section, the detection efficiency of a two-photon microscope (equipped with appropriate widefield detectors) is about 10,000 times better than that of a confocal microscope. Laser light source Collimator Beam path of the excitation and emission light in a confocal laser scanning microscope. The confocal pinhole screens off fluorescence signals from non-focal planes and thus Focal plane permits optical sections to be made. Main dichroic beamsplitter Scanning mirrors Objective Specimen
7 The limits of confocal microscopy The confocal microscope reaches its limits when it comes to imaging fluorescent structures in deeper layers of tissue. The deeper the target spot, the greater are the absorption and scattering of visible light. At a certain depth, the excitation intensity is no longer sufficient to create a fluorescence signal that provides a distinct image of the structure of interest. The same cells excited with a femtosecond laser at 890 nm. Excitation is confined to the focal spot. As a result, the fine cell processes are distinctly visible. Femtosecond lasers excite the fluorochrome only at the focus. Laser light source Collimator Energy Energy diagram of fluorescence generation with multiphoton excitation. Detector Open pinhole in the confocal plane Main dichroic beamsplitter Scanning mirrors Nonlinear optics (NLO): Excitation on the spot A femtosecond laser used on the LSM 510 NLO or LSM 510 META NLO eliminates most of the restrictions of the confocal microscope. This laser emits light in the near infrared spectral range (between 700 and 1100 nm), in ultrashort pulses with a pulse energy of up to 170 kw and a repetition rate of 76 to 90 MHz. Spot-focused on the object of interest, the laser beam excites fluorescence if at least two photons are absorbed by a fluorochrome molecule within less than a femtosecond (10 15 seconds). This effect varies exponentially with the intensity of the excitation light. The probability of excitation is maximum at the objective focus, outside of which it drops extremely fast. Thus, the fluorescent light created by this nonlinear effect exclusively derives from the focal spot. Therefore, the entire light emitted can be utilized for imaging the signal. Beam path of the excitation and emission light in a multiphoton microscope. Excitation confined to the focal spot makes a confocal pinhole Focal plane for generating optical sections superfluous. Objective Specimen 5
8 System Components Efficiency by Perfect Interaction The LSM 510 NLO and LSM 510 META NLO microscopes implement multiphoton microscopy at its best. With their exactly matched components, they decisively expand your experimental capabilities. Scanning module The scanning module is the core of the LSM 510 NLO and LSM 510 META NLO. It contains motor-driven collimators, scanning mirrors, individually adjustable and positionable pinholes, and highly sensitive detectors. All these components are arranged to ensure optimum specimen illumination and efficient collection of reflected or emitted light. Detectors 1) Descanned: The LSM 510 NLO has up to four detectors with individual pinholes. The LSM 510 META NLO permits the emission signal to be split up according to spectral properties. With the innovative META detector you can identify fluorochromes by their spectral signature either online or off-line. 2) Non-Descanned: Non-descanned detectors are an indispensable option for detecting heavily scattered fluorescence signals, for example in brain tissue. A motor-driven filter wheel allows you to detect two fluorescence signals simultaneously. You can use up to four detectors, two each in the reflected-light and transmitted-light beams. Laser module Beam path (schematic) in the LSM 510 NLO scanning module To excite fluorescent structures, the LSM 510 NLO and LSM 510 META NLO instruments provide laser lines in a range of nm. The laser is attenuated by means of acousto-optical tunable filters (AOTF). The additional coupling of a femtosecond laser, either direct or via an optical fiber, turns the instrument into a multiphoton microscope. In the fiber-coupled configuration, optimum pulse width setting at the specimen is ensured by a grating dispersion compensator (GDC) and a post fiber compressor (PFC); laser attenuation is by an acousto-optical modulator
9 Control of the femtosecond laser by the LSM software Microscopes The high-grade Zeiss research microscopes guarantee unsurpassed image quality and optical perfection. You can choose between Axioplan 2 imaging MOT, Axioskop 2 FS MOT and Axiovert 200M. All are fully motor-driven, LSM-softwarecontrolled, and equipped with ICS optics. Objectives Objectives especially designed for use with NIR light provide the best possible combination of resolving power, aperture and working distance. We highly recommend the IR Achroplan series. Electronics module The LSM 510 NLO and LSM 510 META NLO are controlled by digital signal processors (DSP). They bring about fast, flexible synchronization of scanners, AOTF, AOM and detectors. This enables such sophisticated functions as Multitracking; Spline Spot, and Step Scan; Region of Interest (ROI) Scan; and Spot, Z, and ROI Bleaching independent of the scan position. Moreover, this technology permits the implementation of new scanning functions through simple software upgrades. Beam path (schematic) in the LSM 510 META NLO scanning module Control computer and software With the PC featuring a powerful processor and the LSM software you can control all system components, including wavelength tuning for some types of directly-coupled femtosecond lasers, making them substantially easier to handle. In addition to the acquisition parameters, the software also saves the laser settings, so that the operating variables for a particular experiment can be reproduced in a later experiment with speed, ease and certainty Optical fibers 2 Motor-driven collimators 3 Beam combiner 4 Main dichroic beamsplitter 5 Scanning mirrors 6 Scanning lens 7, 8, 9 Secondary dichroic beamsplitters 10 Pinholes 11 Emission filters 12 Photomultiplier tubes 13 META detector 14 Neutral density filter 15 Monitor diode 16 Fiber outcoupling
10 Femtosecond Laser and NDD Exploring the Depth The structures and functions of biological systems are increasingly studied on close-to-life research models, including intact animals. High-resolution imaging techniques, however, usually have to rely on thin tissue sections, which yield limited information. Multiphoton microscopy adds a decisive tool to your experimental capabilities. Even in a living animal, fluorescence-labeled cellular structures at depths up to 500 µm can be precisely located and exactly imaged. PD Dr. F. Kirchhoff, Max Planck Institute of Experimental Medicine, Göttingen, Germany Analysis of highly branched processes of EGFP-positive astrocytes at high spatial and temporal resolution deep in acutely isolated tissue sections is an easy task with the Zeiss LSM 510 NLO. The combination of the LSM scanhead, Axioskop 2 FS MOT, Coherent Ti:Sa laser and the straightforward software works from scratch. It is a faithful piece of equipment we rely on. Non-descanned detectors collect the fluorescence signal before the scanning lens. Laser light source Detector Collimator Scanning mirrors Detector Long-pass filter Objective Focal plane Specimen Detector Short-pass filter Detector Non-descanned detectors (NDDs), arranged at the optimum microscope port, detect heavily scattered fluorescence signals from deep tissue layers. Mirror 8
11 z x x z Fluorescence signals generated by femtosecond lasers are precisely detected by non-descanned detectors at much greater depths compared to the detectors in the scanning module (left: descanned; right: non-descanned). GFP-expressing astrocytes, 300 µm deep in a fixed brain section taken from a genetically engineered mouse, imaged with detectors in the scanning module (left) and with a non-descanned detector (right). Specimen: PD Dr. F. Kirchhoff, Max Planck Institute of Experimental Medicine, Göttingen, Germany. The proper light source and the right detectors The efficient generation of fluorescence signals by visible excitation light is drastically impaired in deeper tissue layers due to light absorption and scattering. The femtosecond laser, by comparison, efficiently excites fluorescent dyes even in regions several hundreds of µm below the surface. Exciting fluorescence deep down in a tissue is one matter; detecting it is another. Where the fluorescent signal is emitted deep in tissue and subjected to heavy scattering, its registration calls for direct detectors located in front of the scanning lens and the confocal pinhole. Our systems provide up to four non-descanned detectors (NDDs) arranged in the reflected-light and transmitted-light beam paths of the microscope. This efficient method of detecting the emitted light has a higher signal-tonoise ratio and supplies more detail information. 9
12 Signal Processor and Laser Attenuation For Intelligent Scanning Strategies With their variety of scanning strategies, the LSM 510 NLO and LSM 510 META NLO are fundamental tools for many experimental approaches. Digital signal processors (DSPs) control the settings for optical manipulation and image acquisition, adapted to the respective experiment. LSM software and DSPs control all system functions. Two independently controllable scanning mirrors enable such different scanning strategies as point illumination of different sites in the specimen or scanning along a freely defined line. All parameters for image acquisition like detector voltage and the attenuation of all lasers are exactly synchronized. Some of the many scanning strategies provided are the definition of different laser settings depending on the focal plane in 3D imaging (Auto Z Brightness Correction), the limitation of specimen illumination and image acquisition to real regions of interest defined with single-pixel accuracy (RealROI), and bidirectional scanning (Dual Direction Scan, DDS). The fast and exact control of laser intensity is decisive also for a high level of specimen preservation. Outside the image acquisition times, the specimen is not subject to any light load. Control of the scanning parameters by the LSM software. Laser intensity attenuation is set gradually in steps between 0.1 and
13 Auto Z Brightness Correction Constant Image Quality over a Wide Depth Range Complete three-dimensional reconstructions of fluorescent structures deliver valuable information about the architecture of cells and tissues. Once defined, the settings of laser intensity, detector voltage and offset for the beginning and end of the Z stack are saved. In the recording of extended Z stacks, the fluorescence signal gets fainter as the focus is moved to greater depth. To compensate the loss, conventional techniques require the detector voltage and laser intensity settings for the various optical sections to be adapted manually. The Auto Z Brightness Control function of the LSM software does away with manual adjustment. You only need to define the settings for the first and the last optical section, and you will get a stack of images with uniformly intense fluorescence signals. 3D reconstructions of such stacks supply highly informative spatial representations of cells or tissues. 3D reconstruction of GFP-expressing astrocytes in the neocortex of a mouse. Specimen: Dr. J. Hirrlinger, Max Planck Institute of Experimental Medicine, Göttingen, Germany. 11
14 Region of Interest (ROI) Arbitrarily Defined Areas Manipulations with living cells such as the bleaching of fluorescent proteins or the photochemical uncaging of biologically active substances have to be done in precisely defined areas to create reproducible data. Such areas, termed ROIs, are defined in the LSM software with single-pixel accuracy. You can define a number of ROIs of varied sizes and with any outlines. Select one or several of them and the laser light will only irradiate the areas selected. This flexible technique supports many experimental tasks such as Fluorescence Recovery after Photobleaching (FRAP), Fluorescence Loss in Photobleaching (FLIP), photochemical uncaging of substances, or photoactivation. Moreover, the MultiTimeSeries module, combined with a motor-driven XY stage, allows the completely automatic irradiation of individual regions with varied laser lines and light intensities. ROIs can be defined differently for different scanning configurations. In long-time experiments, the Auto Focus function keeps the region to be examined in the focal plane. If a Region of Interest (ROI) is defined in the specimen image, only this region will then be illuminated. Signal detection from several ROIs is effected by a single image acquisition action. Bleaching the nucleus region of GFP-expressing COS 7 cells. The area to be irradiated is defined with an ROI. Specimen: A. Böhmer, Friedrich Schiller University of Jena, Germany. ROIs are defined in the image by means of drawing tools or by entering their exact positions, and saved. 12
15 Spot Scan and Spot Bleach Detection of the Smallest Possible Area To capture fast processes taking place in small regions of a cell, the changing fluorescence signal must be imaged with a high temporal resolution. For that purpose, the Spot Scan and Spot Bleach functions reduce the detection area to the smallest possible size. In the Spot Scan and Spot Bleach modes, the scanning mirrors are parked in a defined position, so that only a spot in the specimen is illuminated. The fluorescent signals at the focal spot are recorded at intervals of a few microseconds. If you want to combine specimen imaging with bleaching, you may select differing positions in the specimen. In this way you can track, for example, the bleaching of the fluorescent label or the photolytic uncaging of a chemically modified messenger substance in the immediate vicinity with the appropriate temporal resolution. Crosshairs define the positions for bleaching (green crosshairs) and signal recording (red crosshairs) in the image. In the Spot mode, you can repeatedly bleach a position different from that defined for signal recording. The intensity graph informs about the effect of bleaching on the fluorescent protein in the position of signal detection. 13
16 Z Bleach Optimum Use of Focal Excitation The femtosecond laser excites fluorochromes exclusively in the focal plane. With the Z Bleach function of the LSM software, this property can be optimally utilized for different experimental approaches. You can define different planes for the optical manipulation of specimens and the detection of the emission signals. Fluorescence Recovery after Photobleaching (FRAP) is a method used in the investigation of transport processes between cells or cell compartments. For highly informative results, it is important to limit the area to be manipulated not only in the X and Y but also in the Z direction, as this avoids the unintentional bleaching of non-defined areas. In experiments that track the loss of fluorescence in unbleached areas (FLIP), the LSM software makes it possible to select different planes for the bleaching action and for fluorescence change tracking. Within a time series, experimental routine procedures can be set up in a very quick and easy way and then run automatically. As a result you obtain image series that supply quantitative data immediately. The method also permits targeted illumination of individual cells in a tissue in order to label them by the uncaging of a fluorochrome or by photoactivation of PA-GFP. The activation can be repeated time and again after a defined number of images is acquired. The predefined Z plane is moved into focus during bleaching. Definition of a ROI for bleaching. z x Axial extension of the bleached area in confocal scanning microscopy (left) and multiphoton microscopy (right). 14
17 1,5 m 1,5 m Prof. Y. Oshima, Kyushu University, Fukuoka, Japan Our LSM 510 NLO is very useful in morphological analysis of deeper portions of GFP-expressing C. elegans worms especially in that ROI (Region Of Interest) and Time Lapse programs enable us to perform FRAP and FLIP by which we can get in vivo molecular data that we cannot get with other methods. 3 m 3 m 4,5 m 4,5 m This brings about the complete activation or uncaging, respectively, within a cell, as fluorochromes not irradiated will gradually diffuse into the focal plane. For the user of an LSM 510 NLO or LSM 510 META NLO instrument, the property of the femtosecond laser to excite a fluorochrome only within a volume of less than a femtoliter (1 µm 3 ) is harnessed to provide an easy-to-handle tool. 6 m 6 m 7,5 m 7,5 m Drosophila embryo labeled for glial cells (CY3, red), neurons (FITC, green), and nuclei (DAPI, blue). Excitation with 780 nm, detection with META detector in the Online Fingerprinting mode. After the experiment, the labeling of the glial cells has bleached within a confined axial range. In the orthogonal view, the Z extension of the bleached region can be determined. 15
18 Visible Light and Near Infrared A Clever Combination With motor-driven collimators, the excitation planes of near infrared and visible light can be precisely matched. The mere availability of lasers emitting visible and infrared light is one thing; making the best possible use of these light sources is another. For the latter purpose, the LSM 510 NLO and LSM 510 META NLO instruments feature motor-driven collimators, which precisely match the excitation planes for different wavelengths. Establishing the optimum collimator position by means of a fluorescent bead. The collimator for the near infrared excitation range is shifted step by step to make the visible light and near infrared excitation planes coincide. Computer-controlled, servomotor-driven collimators ensure that the excitation with visible and infrared light takes place in precisely the same specimen plane, even if the excitation wavelengths are far apart. Collimator settings are optimized for each objective and saved together with the image data. A desired setting can be activated any time with the ReUse function. The high output power of the femtosecond laser one watt plus is suitable, e.g., for the targeted damaging of cell processes. Thanks to the collimators, you can subsequently record the fluorescence signal of the manipulated structures with any desired excitation laser. This technique also enables you to choose from an exceptionally broad range of fluorochromes. For example, you can use DAPI to label a cell nucleus and excite it with the femtosecond laser. Simultaneous use of femtosecond and visible light lasers. In a triple-stained specimen, two fluorescence signals are exited by visible light (green and red), and the third one by infrared light (blue). 16
19 META Detection Fast, Positive Separation of Fluorescence Signals The META detector separates mixed fluorescence signals and positively assigns the light emissions to the fluorochromes from which they originate. The method (patent pending) supplies images of substantially higher information content. A femtosecond laser often excites more than one of the fluorochromes applied; the emitted mix may even contain autofluorescence. The LSM 510 META NLO will identify marker dyes and autofluorescence by their spectral properties. The method of Emission Fingerprinting clearly separates the fluorescent components from each other. Multiple labeling clearly separated Many of the dyes used in conventional fluorescence microscopy are also suitable for multiphoton microscopy. As many excitation spectra overlap considerably in the near infrared range, it often happens that several fluorochromes are excited simultaneously. The same applies to autofluorescent substances. The phenomenon makes it difficult to separate labeled structures with filters. The META detector overcomes this handicap in a technically elegant and plausible manner: A highly efficient optical grating projects the entire fluorescence spectrum onto the 32 channels of the META detector. Thus, the complete spectral signature is recorded for every pixel of the scanned image and subsequently used for digital separation into the signal components. The Emission Fingerprinting function detects autofluorescence separately which can then be filtered out or used for additional information. Salivary gland of a cockroach (P. americana) labeled for dopamine (CY3, red) and actin (Oregon Green Phalloidine, green), autofluorescence (blue). Specimen: PD Dr. O. Baumann, University of Potsdam, Germany. 17
20 Excitation Fingerprinting The Expert Way of Using Excitation Spectra With the femtosecond laser, many conventional flu- Record orochromes are excitable over a wavelength range excitation spectra... of more than 100 nm. The LSM 510 NLO and LSM 510 META NLO microscopes will determine the optimum excitation wavelength quickly and reliably. Moreover, Excitation Fingerprinting uses excitation spectra to positively identify fluorescence signals. Finding the optimum excitation wavelength for a fluorochrome used to be a time-consuming job. With the LSM 510 NLO and LSM 510 META NLO and a software-controlled femtosecond laser, excitation spectra tailored to a particular specimen can be recorded quickly and efficiently. Image acquisition at varied wavelengths supplies what is x z called a lambda stack, from which excitation spectra are obtained. Real spectra are produced with a uniform laser intensity in the specimen plane, which is y The images of an excitation lambda stack are excited with different wavelengths. implemented by an appropriate setting of the AOM. Calibration curves for the AOM are established by an automatic procedure using a power meter, and saved. The normalized Excitation spectra show signal intensities as a function of excitation wavelength CFP GFP YFP calibration curves are then used in the recording of lambda stacks. Intensity) Wavelength Calibration of the AOM is done with the Excitation Fingerprinting macro. The result is a constant intensity level of the laser light in the specimen plane, irrespective of wavelength. The macro is also used to produce excitation lambda stacks. The user defines the excitation wavelength range, the spacing between the various wavelengths used, and the appropriate AOM calibration curve. 18
21 ... and use them for Excitation Fingerprinting 720 nm 730 nm 740 nm The excitation spectra not only greatly facilitate the definition of the optimum excitation wavelength of one or several fluorochromes, but are also helpful in distinguishing between fluorescence signals with overlapping emission spectra. The Linear Unmixing function of the LSM software uses excitation spectra to reliably compute the shares of the fluorochromes in the specimen. The Automatic Component Extraction function (ACE) can be used for the identification of fluorescent dyes with but short topographic overlaps. Nondescanned detectors are recommended to detect fluorescence signals deep in heavily scattering specimens. If overlapping, these signals, too, can be exactly separated. 750 nm 760 nm 780 nm 790 nm Excitation Fingerprinting separates widely overlapping emission signals using their excitation spectra. Intensity nm Excitation wavelength Drosophila retina, labeled for actin (Alexa Fluor 568 Phalloidine). Excitation Fingerprinting clearly separates autofluorescence and emission signals. Specimen: PD Dr. O. Baumann, University of Potsdam, Germany 19
22 LSM 510 NLO Specification Microscopes Models Z drive HRZ 200 (option) XY stage (option) Accessories Upright: Axioplan 2 imaging MOT, Axioskop 2 FS MOT; inverted: Axiovert 200 M BP (Base Port) or SP (Side Port) DC motor with optoelectronic coding, smallest increment 25 or 50 nm; fast piezo focusing accessory High-precision galvanometric fine focusing stage, total lift 200 µm, smallest increment <10 nm Motor-driven XY scanning stage, with Mark & Find (XYZ) and Tile Scan functions, smallest increment 1 µm AxioCam digital microscope camera, incubation chambers, micromanipulators, etc. Scanning module Models Scanner Scanning resolution Scanning speed 2 or 3 confocal channels 2 independent galvanometric scanning mirrors, DSP-controlled, for ultrafast line and vertical flyback 4 x1 to 2048 x 2048 pixels, also for several channels, continuously adjustable 13 x 2 speed stages; up to 5 frames/s with 512 x 512 pixels (up to 77 frames/s with 512 x32 pixels); min ms for a line of 512 pixels Scanning zoom 0.7x to 40x, digital, variable in steps of 0.1 Scanning rotation Scanning field Pinholes Detection Data depth Free 360 rotation, variable in steps of 1 degree, free XY offset Max. diagonal 18 mm in the intermediate image plane, homogeneous illumination Up to 4 pinholes for each confocal channel, adjustable in size and position Up to 4 confocal channels for fluorescence/reflectance with short, efficient beam paths, each with a high-sensitivity PMT detector. Options: Transmitted-light channel with PMT Monitor diode for measuring the excitation intensity Non-descanned detectors (NDD) for multiphoton microscopy up to 4 detectors for reflectedand transmitted-light fluorescence; max. 2 channels each in the reflected- and transmitted-light beam paths Selectable between 8 bit and 12 bit Laser module VIS laser module Multiphoton module (option) Polarization-preserving single-mode fiber, temperature-stabilized VIS-AOTF for simultaneous intensity control of up to 6 visible-light laser lines, switching time < 5 µs; AOTF reprogramming via the LSM software. Ar laser (458, 477, 488, 514 nm), 30 mw; HeNe laser (543 nm), 1 mw; HeNe laser (633 nm), 5 mw (end-of-lifetime specification) Direct or fiber coupling of pulsed Ti:Sa lasers into the scanning module; various makes are supported. Grating Dispersion Compensator (GDC) and Post Fiber Compressor (PFC) for optimum pulse shaping. Fast change of laser intensity by means of AOM. Objectives optimized for use in the NIR range Electronics module LSM 510 Control Computer Control of the microscope, the VIS and NLO laser modules, the scanning module and further accessories. Monitoring of data acquisition and synchronization by a Digital Signal Processor (DSP). Data exchange between DSP and computer via ultra-wide SCSI port High-end PC with generous working memory and hard disk storage capacity; ergonomic high-resolution monitor or TFT flat-panel display, many accessories; Windows 2000/NT 4.0 operating system with multi-user capability 20
23 Standard software System configuration ReUse function Acquisition modes Auto Z Crop RealROI Scan ROI Bleach Spline Scan Multitracking Presentation Image analysis Image operations Image archiving, export, import Convenient control and configuration of all motorized microscope functions, of the laser and scanning modules; saving and restoration of application-specific configurations Restoration of acquisition parameters with one mouse click Spot, Line/Spline, Frame, Z Stack, Time Series and combinations: XY, XYZ, XYt, XYZt, XZ, Xt, XZt, Spot-t. On-line computation and presentation of ratio images. Averaging and summation (linewise or framewise, configurable). Step scan (for higher frame rates, configurable) On-line adaptation of Z stack acquisition parameters for uniform brightness distribution Convenient selection of scanning ranges (zoom, offset, rotation simultaneously) Scanning of up to 99 ROIs (Regions of Interest) of any shape, with pixel-accurate laser blanking Localized photobleaching of up to 99 bleaching ROIs for applications such as FRAP (Fluorescence Recovery After Photobleaching) or uncaging Scanning along a free-hand defined line Acquisition of multiple fluorescences; minimized signal crosstalk (bleeding) by fast change of excitation lines Orthogonal view (XY, XZ, YZ in a single presentation), cut view (3D section made under a freely definable spatial angle), 2.5D view for time series of line scans, projections (stereo, maximum, transparent) for single frames and series (animations), depth coding (pseudo-color presentation of height information). Brightness and contrast adjustments; off-line interpolation for Z stacks, selection and modification of color lookup tables (LUT), drawing functions for documentation Modern tools for colocalization and histogram analysis with diverse parameters and options, profile measurement of straight lines and curves of any shape, measurement of lengths, angles, areas, intensities, etc. Addition, subtraction, multiplication, division, ratio, shift, filters (low-pass, median, high-pass, etc.; user-definable) LSM image database with convenient functions for managing images together with their acquisition parameters; multiprint function for creating assembled image and data views; more than 20 file formats (TIF, BMP, JPG, PSD, PCX, GIF, AVI, Quicktime ) for compatibility with all common image processing programs Software Options LSM Image VisArt 3D Deconvolution Multiple Time Series 3D for LSM Physiology Topography Macro Edit Fast 3D and 4D reconstruction and animation (silhouette projection, transparent projection, surface rendering) Image restoration based on computed point spread functions (Nearest Neighbor, Maximum Likelihood, Constraint Iterative) Assembled time series with change of application-specific configurations, autofocus and bleaching functions 3D reconstruction, thresholding, processing and measurement (surface area, volume, particle count) Analysis of time series, graphical mean-of-roi analysis, on- and off-line display and calibration of ion concentrations Visualization of 3D surfaces (fast rendering modes) plus measurement functions (roughness, waviness, surface area, volume) Acquisition and editing of routines for the automation of scanning and analysis functions Image Browser Free software Presentation, editing, archiving, printing and export/import of LSM 5 images 21
24 LSM 510 META NLO Specification Microscopes Models Z drive HRZ 200 (option) Upright: Axioplan 2 imaging MOT, Axioskop 2 FS MOT Inverted: Axiovert 200 M BP (Base Port) or SP (Side Port) DC motor with optoelectronic coding, smallest increment 25 or 50 nm; fast piezo focusing accessory High-precision galvanometric fine focusing stage, total lift 200 µm, smallest increment <10 nm XY stage (option) Motor-driven XY scanning stage, with Mark & Find (XYZ) and Tile Scan functions, smallest increment 1 µm Accessories (options) AxioCam digital microscope camera, incubation chambers, micromanipulators, etc. Scanning module Models Scanner Scanning resolution Scanning speed META scanning module with 2 single-channel detectors and 1 polychromatic multichannel detector (each genuinely confocal with selected, high-sensitivity PMTs), prepared for lasers from VIS to NIR 2 independent galvanometric scanning mirrors, DSP-controlled, for ultrafast line and vertical flyback 4 x1 to 2048 x 2048 pixels, also for several channels, continuously adjustable 13 x 2 speed stages; up to 5 frames/s with 512 x 512 pixels (up to 77 frames/s with 512 x32 pixels); min ms for a line of 512 pixels Scanning zoom 0.7x to 40x, digital, variable in steps of 0.1 Scanning rotation Scanning field Pinholes Detection META detector Data depth Free 360 rotation, variable in steps of 1 degree, free XY offset Max. diagonal 18 mm in the intermediate image plane, homogeneous illumination Pinholes for each epi-illumination channel (single-channel detector or META multichannel detector), individual adjustments of size and position, preadjusted Standard: 3 confocal epi-illumination channels simultaneously (META detector + 2 single-channel detectors), each with a high-sensitivity PMT detector. Options: Transmitted-light channel with PMT Monitor diode for measuring the excitation intensity Non-descanned detectors (NDD) for multiphoton microscopy up to 4 detectors for reflectedand transmitted-light fluorescence; max. 2 channels each in the reflected- and transmitted-light beam paths Polychromatic 32-channel detector for fast acquisition of lambda stacks and metatracking; simultaneous acquisition in up to 8 channels; also in combination with time series Selectable between 8 bit and 12 bit Laser module VIS laser module Multiphoton module (option) Polarization-preserving single-mode fiber, temperature-stabilized VIS-AOTF for simultaneous intensity control of up to 6 visible-light laser lines, switching time < 5 µs; AOTF reprogramming via the LSM software. Ar laser (458, 477, 488, 514 nm), 30 mw; HeNe laser (543 nm), 1 mw; HeNe laser (633 nm), 5 mw (end-of-lifetime specification) Direct or fiber coupling of pulsed Ti:Sa lasers into the scanning module; various makes are supported. Grating Dispersion Compensator (GDC) and Post Fiber Compressor (PFC) for optimum pulse shaping. Fast change of laser intensity by means of AOM. Objectives optimized for use in the NIR range Electronics module LSM 510 Control Computer Control of the microscope, the VIS and NLO laser modules, the scanning module and further accessories. Monitoring of data acquisition and synchronization by a Digital Signal Processor (DSP). Data exchange between DSP and computer via ultra-wide SCSI port High-end PC with generous working memory and hard disk storage capacity; ergonomic high-resolution monitor or TFT flat-panel display, many accessories; Windows 2000/NT 4.0 operating system with multi-user capability 22
25 Standard software System configuration ReUse function Aquisition modes Auto Z Crop RealROI Scan ROI Bleach Spline Scan Multitracking Metatracking Lambda Scan Emission Fingerprinting Presentation Image analysis Image operations Image archiving, export, import Convenient control and configuration of all motorized microscope functions, of the laser and scanning modules; saving and restoration of application-specific configurations Restoration of acquisition parameters with one mouse click Spot, Line/Spline, Frame, Z Stack, Lambda Stack, Time Series and combinations: XY, XYZ, XYt, XYZt, XZ, Xt, XZt, Spot-t, Xλ, XYλ, XYZλ, XYtλ, XYZtλ, XZλ, Xtλ, XZtλ. On-line computation and presentation of ratio images. Averaging and summation (linewise or framewise, configurable). Step scan (for higher frame rates, configurable) On-line adaptation of Z stack acquisition parameters for uniform brightness distribution Convenient selection of scanning ranges (zoom, offset, rotation simultaneously) Scanning of up to 99 ROIs (Regions of Interest) of any shape, with pixel-accurate laser blanking Localized photobleaching of up to 99 bleaching ROIs for applications such as FRAP (Fluorescence Recovery After Photobleaching) or uncaging Scanning along a free-hand defined line Acquisition of multiple fluorescences; minimized signal crosstalk (bleeding) by fast change of excitation lines Extension of multitracking by fast electronic change of detection channels, even with overlapping bandpasses, ensures optimum signal detection (only with META detection module) Fast acquisition of image stacks with spectral information for every pixel (only with META detection module) Technique for generating crosstalk-free multiple-fluorescence images with simultaneous excitation, online or offline unmixing, automatic or interactive definition of reference spectra (only with META detection module) Orthogonal view (XY, XZ, YZ in a single presentation), cut view (3D section made under a freely definable spatial angle); 2.5D view for time series of line scans. Projections (stereo, maximum, transparent) for single frames and series (animations). Depth coding (pseudo-color presentation of height information). Brightness and contrast adjustments. Off-line interpolation for Z stacks. Selection and modification of color lookup tables (LUT). Drawing functions for documentation Modern tools for colocalization and histogram analysis with diverse parameters and options, profile measurement of straight lines and curves of any shape, measurement of lengths, angles, areas, intensities, etc. Addition, subtraction, multiplication, division, ratio, shift, filters (low-pass, median, high-pass, etc.; user-definable) LSM image database with convenient functions for managing images together with their acquisition parameters. Multiprint function for creating assembled image and data views. More than 20 file formats (TIF, BMP, JPG, PSD, PCX, GIF, AVI, Quicktime ) for compatibility with all common image processing programs Software Options LSM Image VisArt 3D Deconvolution Multiple Time Series 3D for LSM Physiology Topography Macro Edit Fast 3D and 4D reconstruction and animation (silhouette projection, transparent projection, surface rendering) Image restoration based on computed point spread functions (Nearest Neighbor, Maximum Likelihood, Constraint Iterative) Assembled time series with change of application-specific configurations, autofocus and bleaching functions 3D reconstruction, thresholding, processing and measurement (surface area, volume, particle count) Analysis of time series, graphical mean-of-roi analysis, on- and off-line display and calibration of ion concentrations Visualization of 3D surfaces (fast rendering modes) plus measurement functions (roughness, waviness, surface area, volume) Acquisition and editing of routines for the automation of scanning and analysis functions Image Browser Free software Presentation, editing, archiving, printing and export/import of LSM 5 images 23
26 l 1 LSM 510 NLO and LSM 510 META NLO System Overview NLO kit for fiber coupling VIS/NLO-Scan module LSM 510 Ar-laser, 458, 477, 488, 514 nm, 30 mw NLO kit for direct coupling Upgrade kits LSM 510 to LSM 510 META HeNe laser, 543 nm, 1mW HeNe laser, 633 nm, 5 mw Laser module VIS VIS AOTF (6 channels) VIS/NLO Scan module LSM 510 META Fiber decoupling, channel 4 Options: -Fluorescence Correlation Spectroscopy (FCS) -Fluorescence Lifetime Imaging Microscopy (FLIM) Actively vibration-absorbed system table for LSM 5 Table surface 30" x 30" Monitor 21" (50 cm) LCD TFT-Flatscreen 18" Host computer Large LSM 5 system table width 1500 mm, height 780 mm, depth 800 mm with vibration absorption Granite slab Small LSM 5 system table width 650 mm, height 780 mm, depth 800 mm with vibration absorption Granite slab System table NLO with active absorption width 1800 mm, height 750 mm, depth 1400 mm System table NLO with active absorption width 1200 mm, height 750 mm, depth 1400 mm 24
27 1:1 70 Coarse K Piezo objective focus Illuminator N HBO 103 Power supply unit N HBO 103 VIS/NLO Scan module LSM 510 META HRZ 200 fine focusing stage AxioCam HRm AxioCam HRc Non Descanned Detection kit Epifluorescence motorized NDD module with shutter Non Descanned Detection modul I O Fine Axioplan 2 imaging MOT Scanning stage DC100x100 (65x50 mm) 100 HAL lamp housing with collector, lamp mount Halogen lamp 12 V 100 W HRZ 200 fine focusing stage Switching mirror mot Axiovert 200 M SP Axiovert 200 M BP Several solutions for incubation will be offered. Transmitted-light channel for LSM 5 Scanning stage DC 120x100 with mounting frame VIS/NLO Scan module LSM 510 META 100 HAL lamp housing Lamp mount Halogen lamp 12 V 100 W Non Descanned Detection kit motorized NDD module with shutter VIS/NLO Scan module LSM 510 META AxioCam HRm AxioCam HRc Non Descanned Detection modul Motor control MCU 28 2-axes control panel AxioCam HRm AxioCam HRc 100 HAL lamp housing with collector, lamp mount Halogen lamp 12 V 100 W Power supply 12 V DC 100 W, stabilized Switching mirror mot ECU for LSM 510 / LSM 510 META Transmitted-light channel for LSM 5 25
28 LSM 510 NLO and LSM 510 META NLO Functionality at a Glance Automatic Component Extraction Statistical procedure for identifying individual fluorochrome spectra in a lambda stack. Auto Z Brightness Correction Procedure for compensating signal loss in deep tissue layers in Z stack acquisition, to obtain signals of equal intensity from the different planes. Emission Fingerprinting* (patent pending) Method of the LSM 510 META NLO for the acquisition, analysis and separation of emission signals in multiple fluorescence images; also applicable to widely overlapping spectra. Excitation Fingerprinting (patent pending) Method for the acquisition, analysis and separation of emission signals from multiple-fluorescence-labeled specimens by means of excitation spectra. Lambda Stack Image stack with information in the X, Y and λ dimensions; combinable with Z and/or time series; for the determination of spectral signatures anywhere in the specimen. Linear Unmixing Mathematical procedure for the spectral deconvolution of multiple emission signals. Metatracking* Scanning mode in the LSM 510 META NLO, similar to Multitracking, but supplemented by fast switching between detection settings. RealROI Scan Scanning mode in which freely defined areas of the specimen are excited and imaged; maximum specimen preservation thanks to exact blanking of the laser lines outside the selected areas. ROI Bleaching Defined bleaching of several freely defined specimen areas, used, e.g., in FRAP or uncaging experiments. Spline Scan Scanning along a freely defined line for recording fast (physiological) changes, e.g. along neuron processes. Spot Scan Scanning mode in which the signal intensity in a confocal spot is tracked with extremely high temporal resolution. Spot Bleach Bleaching mode used in time series, in which a defined specimen spot is bleached and the signal recorded at a different spot with extremely high temporal resolution. Step Scan Fast overview scan, in which intermediate lines are interpolated. Tile Scan Acquisition of the image of a large object as a mosaic of partial images, to achieve better resolution. Z Bleach Bleaching mode in which the plane of bleaching is defined independent of the image acquisition plane. Multitracking Scanning mode in all models of the LSM 5 family, creates multiple fluorescence images without emission signal crosstalk, thanks to fast switching between excitation and quasi-simultaneous detection. * only for LSM 510 META NLO 26
29 Carl Zeiss Consulting Carl Zeiss Service With their know-how of products and markets, our team will assist you in selecting the system that meets your specific requirements, including components of other makes. After every system installation, our regional specialists will intensively train your operators. Consult us about latest applications to continuously extend the use of the LSM 510 NLO or the LSM 510 META NLO in your research. Carl Zeiss Training Courses To you and your staff, Carl Zeiss offers comprehensive training courses about every aspect of confocal microscopy. Our seminars, whether entry-level or advanced, are always practice-related. In local workshops you will learn everything about current applications and new technical features introduced by Carl Zeiss, as well as gain hands-on experience in how to use the systems most efficiently. Dependable, competent Zeiss staff will install and maintain your system. The new detection module can readily be retrofitted to any LSM 510 NLO system installed, to make up a fully functional LSM 510 META NLO. The existing optical, mechanical and electronic interfaces permit further configuration for methods such as the measurement of molecule interactions by means of FCS (fluorescence correlation spectroscopy). New scanning and analysis methods can be made available quickly and easily through software upgrades.
30 Glossary AOM Acousto Optical Modulator AOTF Acousto Optical Tunable Filter DDS Dual Direction Scan DSP Digital Signal Processor FLIP Fluorescence Loss in Photobleaching FRAP Fluorescence Recovery after Photobleaching GDC Grating Dispersion Compensator GFP Green Fluorescent Protein ICS Infinity Color Corrected System MOT Motor-driven Microscope Model NDD Non-Descanned Detector NLO Nonlinear Optics NIR Near Infrared PFC Post Fiber Compressor PMT Photo Multiplier Tube ROI Region of Interest
31 The History of Multiphoton Microscopy 1931 Maria Goeppert-Mayer (Nobel prize winner in physics, 1963) describes two-photon processes in her doctoral thesis Über Elementarakte mit zwei Quantensprüngen (On Elementary Acts Involving Two Quantum Leaps) (Göttingen): Ann.Phys. 9: The first Carl Zeiss Laser Scanning Microscope. The prototype of the series is now an exhibit at the German Museum, Munich Several publications on experiments with two-photon processes in biological specimens (Lesclaux R, Vsevolodov NN, Berns MW) First publication on two-photon laser scanning microscopy: Denk W, Strickler JH, Webb WW, Science 1990, 248 (4951): The LSM 310 combines confocal laser scanning microscopy with leading-edge PC technology First system of the LSM 510 series Model LSM 510 NLO, prepared for multiphoton microscopy LSM 510 META NLO, most advanced technology plus convenient operation.
32 LSM 510 META NLO and LSM 510 NLO for Multiphoton Microscopy The integration of laser control into the LSM software adds convenient operation to an efficient technology. An instrument combination suitable for many applications in biomedical research. US Patents: , , , , , , , , , , , German Patents: C2, C2, C2, C2, C2, C2, C2, C2, C2, C2, C2 For further information, please contact: Carl Zeiss Advanced Imaging Microscopy Jena GERMANY Phone: Telefax: Subject to change e/12.03
Multifluorescence The Crosstalk Problem and Its Solution
 Multifluorescence The Crosstalk Problem and Its Solution If a specimen is labeled with more than one fluorochrome, each image channel should only show the emission signal of one of them. If, in a specimen
Multifluorescence The Crosstalk Problem and Its Solution If a specimen is labeled with more than one fluorochrome, each image channel should only show the emission signal of one of them. If, in a specimen
LSM 510 META Laser Scanning Microscope Fluorescence Signals Reliably Separated
 Microscopy from Carl Zeiss LSM 510 META Laser Scanning Microscope Fluorescence Signals Reliably Separated Highlights of Laser Scanning Microscopy 1982 The first Laser Scanning Microscope from Carl Zeiss.
Microscopy from Carl Zeiss LSM 510 META Laser Scanning Microscope Fluorescence Signals Reliably Separated Highlights of Laser Scanning Microscopy 1982 The first Laser Scanning Microscope from Carl Zeiss.
Microscopy from Carl Zeiss LSM 710. The Power of Sensitivity. A New Dimension in Confocal Laser Scanning Microscopy
 Microscopy from Carl Zeiss LSM 710 The Power of Sensitivity A New Dimension in Confocal Laser Scanning Microscopy Sensitivity Is the Key Whether it is in live cell imaging, single molecule analysis or
Microscopy from Carl Zeiss LSM 710 The Power of Sensitivity A New Dimension in Confocal Laser Scanning Microscopy Sensitivity Is the Key Whether it is in live cell imaging, single molecule analysis or
Why and How? Daniel Gitler Dept. of Physiology Ben-Gurion University of the Negev. Microscopy course, Michmoret Dec 2005
 Why and How? Daniel Gitler Dept. of Physiology Ben-Gurion University of the Negev Why use confocal microscopy? Principles of the laser scanning confocal microscope. Image resolution. Manipulating the
Why and How? Daniel Gitler Dept. of Physiology Ben-Gurion University of the Negev Why use confocal microscopy? Principles of the laser scanning confocal microscope. Image resolution. Manipulating the
Zeiss 780 Training Notes
 Zeiss 780 Training Notes Turn on Main Switch, System PC and Components Switches 780 Start up sequence Do you need the argon laser (458, 488, 514 nm lines)? Yes Turn on the laser s main power switch and
Zeiss 780 Training Notes Turn on Main Switch, System PC and Components Switches 780 Start up sequence Do you need the argon laser (458, 488, 514 nm lines)? Yes Turn on the laser s main power switch and
Maria Smedh, Centre for Cellular Imaging. Maria Smedh, Centre for Cellular Imaging
 Nonlinear microscopy I: Two-photon fluorescence microscopy Multiphoton Microscopy What is multiphoton imaging? Applications Different imaging modes Advantages/disadvantages Scattering of light in thick
Nonlinear microscopy I: Two-photon fluorescence microscopy Multiphoton Microscopy What is multiphoton imaging? Applications Different imaging modes Advantages/disadvantages Scattering of light in thick
長庚大學共軛焦顯微鏡課程 長庚大學共軛焦顯微鏡課程. Spot light 長庚大學
 長庚大學共軛焦顯微鏡課程 Spot light 長庚大學共軛焦顯微鏡課程 20071030 長庚大學 Basic principle of Laser Scanning Confocal Microscopy The application of LSM 510 META detector Multiphoton microscopy basic principle and introduction
長庚大學共軛焦顯微鏡課程 Spot light 長庚大學共軛焦顯微鏡課程 20071030 長庚大學 Basic principle of Laser Scanning Confocal Microscopy The application of LSM 510 META detector Multiphoton microscopy basic principle and introduction
ADVANCED METHODS FOR CONFOCAL MICROSCOPY II. Jean-Yves Chatton Sept. 2006
 ADVANCED METHODS FOR CONFOCAL MICROSCOPY II Jean-Yves Chatton Sept. 2006 Workshop outline Confocal microscopy of living cells and tissues X-Z scanning Time series Bleach: FRAP, photoactivation Emission
ADVANCED METHODS FOR CONFOCAL MICROSCOPY II Jean-Yves Chatton Sept. 2006 Workshop outline Confocal microscopy of living cells and tissues X-Z scanning Time series Bleach: FRAP, photoactivation Emission
Confocal Microscopy. Kristin Jensen
 Confocal Microscopy Kristin Jensen 17.11.05 References Cell Biological Applications of Confocal Microscopy, Brian Matsumoto, chapter 1 Studying protein dynamics in living cells,, Jennifer Lippincott-Schwartz
Confocal Microscopy Kristin Jensen 17.11.05 References Cell Biological Applications of Confocal Microscopy, Brian Matsumoto, chapter 1 Studying protein dynamics in living cells,, Jennifer Lippincott-Schwartz
Practical work no. 3: Confocal Live Cell Microscopy
 Practical work no. 3: Confocal Live Cell Microscopy Course Instructor: Mikko Liljeström (MIU) 1 Background Confocal microscopy: The main idea behind confocality is that it suppresses the signal outside
Practical work no. 3: Confocal Live Cell Microscopy Course Instructor: Mikko Liljeström (MIU) 1 Background Confocal microscopy: The main idea behind confocality is that it suppresses the signal outside
Microscopy from Carl Zeiss LSM 700. Laser Scanning Microscope. High-End for All Uncompromised Quality and Operating Convenience
 Microscopy from Carl Zeiss LSM 700 Laser Scanning Microscope High-End for All Uncompromised Quality and Operating Convenience 2 3 The New LSM 700 from Carl Zeiss From a specialists system to the high-end
Microscopy from Carl Zeiss LSM 700 Laser Scanning Microscope High-End for All Uncompromised Quality and Operating Convenience 2 3 The New LSM 700 from Carl Zeiss From a specialists system to the high-end
TRAINING MANUAL. Multiphoton Microscopy LSM 510 META-NLO
 TRAINING MANUAL Multiphoton Microscopy LSM 510 META-NLO September 2010 Multiphoton Microscopy Training Manual Multiphoton microscopy is only available on the LSM 510 META-NLO system. This system is equipped
TRAINING MANUAL Multiphoton Microscopy LSM 510 META-NLO September 2010 Multiphoton Microscopy Training Manual Multiphoton microscopy is only available on the LSM 510 META-NLO system. This system is equipped
Quick Guide. LSM 5 MP, LSM 510 and LSM 510 META. Laser Scanning Microscopes. We make it visible. M i c r o s c o p y f r o m C a r l Z e i s s
 LSM 5 MP, LSM 510 and LSM 510 META M i c r o s c o p y f r o m C a r l Z e i s s Quick Guide Laser Scanning Microscopes LSM Software ZEN 2007 August 2007 We make it visible. Contents Page Contents... 1
LSM 5 MP, LSM 510 and LSM 510 META M i c r o s c o p y f r o m C a r l Z e i s s Quick Guide Laser Scanning Microscopes LSM Software ZEN 2007 August 2007 We make it visible. Contents Page Contents... 1
Shreyash Tandon M.S. III Year
 Shreyash Tandon M.S. III Year 20091015 Confocal microscopy is a powerful tool for generating high-resolution images and 3-D reconstructions of a specimen by using point illumination and a spatial pinhole
Shreyash Tandon M.S. III Year 20091015 Confocal microscopy is a powerful tool for generating high-resolution images and 3-D reconstructions of a specimen by using point illumination and a spatial pinhole
MULTIPHOTON MICROSCOPY. Matyas Molnar Dirk Pacholsky
 MULTIPHOTON MICROSCOPY Matyas Molnar Dirk Pacholsky Information Information given here about 2 Photon microscopy were mainly taken from these sources: Background information on 2-Photon microscopy: http://micro.magnet.fsu.edu/primer/techniques/fluorescence/multiphoton/
MULTIPHOTON MICROSCOPY Matyas Molnar Dirk Pacholsky Information Information given here about 2 Photon microscopy were mainly taken from these sources: Background information on 2-Photon microscopy: http://micro.magnet.fsu.edu/primer/techniques/fluorescence/multiphoton/
LSM 510 META in Chang Gung University
 Content LSM 510 META in Chang ung University LSM 510 META 路 理 The features and applications of LSM 510 META 01-09 Introduction of the hardware 10-12 Fluorescence observation in conventional microscope
Content LSM 510 META in Chang ung University LSM 510 META 路 理 The features and applications of LSM 510 META 01-09 Introduction of the hardware 10-12 Fluorescence observation in conventional microscope
Zeiss 880 Training Notes Zen 2.3
 Zeiss 880 Training Notes Zen 2.3 1 Turn on the HXP 120V Lamp 2 Turn on Main Power Switch Turn on the Systems PC Switch Turn on the Components Switch. 3 4 5 Turn on the PC and log into your account. Start
Zeiss 880 Training Notes Zen 2.3 1 Turn on the HXP 120V Lamp 2 Turn on Main Power Switch Turn on the Systems PC Switch Turn on the Components Switch. 3 4 5 Turn on the PC and log into your account. Start
Training Guide for Carl Zeiss LSM 510 META Confocal Microscope
 Training Guide for Carl Zeiss LSM 510 META Confocal Microscope AIM 4.2 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Turn ON Components and System/PC switches
Training Guide for Carl Zeiss LSM 510 META Confocal Microscope AIM 4.2 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Turn ON Components and System/PC switches
Microscopy from Carl Zeiss
 Microscopy from Carl Zeiss Contents Page Contents... 1 Introduction... 1 Starting the System... 2 Introduction to ZEN Efficient Navigation... 5 Setting up the microscope... 10 Configuring the beam path
Microscopy from Carl Zeiss Contents Page Contents... 1 Introduction... 1 Starting the System... 2 Introduction to ZEN Efficient Navigation... 5 Setting up the microscope... 10 Configuring the beam path
Microscopy from Carl Zeiss LSM 710 NLO. Information in Depth. Innovative Systems for Multiphoton Microscopy
 Microscopy from Carl Zeiss LSM 710 NLO Information in Depth Innovative Systems for Multiphoton Microscopy Providing Support for Progress and Innovation Biomedical sciences represent one of the most important
Microscopy from Carl Zeiss LSM 710 NLO Information in Depth Innovative Systems for Multiphoton Microscopy Providing Support for Progress and Innovation Biomedical sciences represent one of the most important
ZEISS LSM510META confocal manual
 ZEISS LSM510META confocal manual Switching on the system 1) Switch on the Remote Control button located on the table to the right of the microscope. This is the main switch for the whole system including
ZEISS LSM510META confocal manual Switching on the system 1) Switch on the Remote Control button located on the table to the right of the microscope. This is the main switch for the whole system including
Components of confocal and two-photon microscopes
 Components of confocal and two-photon microscopes Internal training 07/04/2016 A. GRICHINE Platform Optical microscopy Cell imaging, IAB, ISdV Plan Confocal laser scanning microscope o o o Principle Main
Components of confocal and two-photon microscopes Internal training 07/04/2016 A. GRICHINE Platform Optical microscopy Cell imaging, IAB, ISdV Plan Confocal laser scanning microscope o o o Principle Main
Boulevard du Temple Daguerrotype (Paris,1838) a busy street? Nyquist sampling for movement
 Boulevard du Temple Daguerrotype (Paris,1838) a busy street? Nyquist sampling for movement CONFOCAL MICROSCOPY BioVis Uppsala, 2017 Jeremy Adler Matyas Molnar Dirk Pacholsky Widefield & Confocal Microscopy
Boulevard du Temple Daguerrotype (Paris,1838) a busy street? Nyquist sampling for movement CONFOCAL MICROSCOPY BioVis Uppsala, 2017 Jeremy Adler Matyas Molnar Dirk Pacholsky Widefield & Confocal Microscopy
Opterra II Multipoint Scanning Confocal Microscope. Innovation with Integrity
 Opterra II Multipoint Scanning Confocal Microscope Enabling 4D Live-Cell Fluorescence Imaging through Speed, Sensitivity, Viability and Simplicity Innovation with Integrity Fluorescence Microscopy The
Opterra II Multipoint Scanning Confocal Microscope Enabling 4D Live-Cell Fluorescence Imaging through Speed, Sensitivity, Viability and Simplicity Innovation with Integrity Fluorescence Microscopy The
LSM 5 EXCITER Laser Scanning Microscope
 Microscopy from Carl Zeiss LSM 5 EXCITER Laser Scanning Microscope Tracking of Cellular Processes We make it visible. The LSM 5 EXCITER from Carl Zeiss is a confocal laser scanning microscope for fundamental
Microscopy from Carl Zeiss LSM 5 EXCITER Laser Scanning Microscope Tracking of Cellular Processes We make it visible. The LSM 5 EXCITER from Carl Zeiss is a confocal laser scanning microscope for fundamental
Leica TCS SP8 Quick Start Guide
 Leica TCS SP8 Quick Start Guide Leica TCS SP8 System Overview Start-Up Procedure 1. Turn on the CTR Control Box, EL6000 fluorescent light source for the microscope stand. 2. Turn on the Scanner Power
Leica TCS SP8 Quick Start Guide Leica TCS SP8 System Overview Start-Up Procedure 1. Turn on the CTR Control Box, EL6000 fluorescent light source for the microscope stand. 2. Turn on the Scanner Power
Non-Descanned FLIM Detection in Multiphoton Microscopes
 Non-Descanned FLIM Detection in Multiphoton Microscopes Abstract. Multiphoton microscopes use a femtosecond NIR laser to excite fluorescence in the sample. Excitation is performed via a multi-photon absorption
Non-Descanned FLIM Detection in Multiphoton Microscopes Abstract. Multiphoton microscopes use a femtosecond NIR laser to excite fluorescence in the sample. Excitation is performed via a multi-photon absorption
DCS-120. Confocal Scanning FLIM Systems. Based on bh s Multidimensional Megapixel FLIM Technology
 DCS-120 Based on bh s Multidimensional Megapixel FLIM Technology Complete Laser Scanning FLIM Microscopes FLIM Upgrades for Existing Conventional Microscopes FLIM with up to 2048 x 2048 pixels Decay curves
DCS-120 Based on bh s Multidimensional Megapixel FLIM Technology Complete Laser Scanning FLIM Microscopes FLIM Upgrades for Existing Conventional Microscopes FLIM with up to 2048 x 2048 pixels Decay curves
DCS-120. Confocal Scanning FLIM Systems. Based on bh s Multidimensional Megapixel FLIM Technology
 Based on bh s Multidimensional Megapixel FLIM Technology Complete Laser Scanning FLIM Microscopes FLIM Upgrades for Existing Conventional Microscopes Multidimensional TCSPC technique High throughput dual-channel
Based on bh s Multidimensional Megapixel FLIM Technology Complete Laser Scanning FLIM Microscopes FLIM Upgrades for Existing Conventional Microscopes Multidimensional TCSPC technique High throughput dual-channel
Training Guide for Leica SP8 Confocal/Multiphoton Microscope
 Training Guide for Leica SP8 Confocal/Multiphoton Microscope LAS AF v3.3 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Turn ON power switch for epifluorescence
Training Guide for Leica SP8 Confocal/Multiphoton Microscope LAS AF v3.3 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Turn ON power switch for epifluorescence
ZEN 2012 SP5 black edition Hotfix 12
 Information about the software ZEN 2012 SP5 black edition Hotfix 12 Software name: ZEN 2012 Service Pack 5 black edition Hotfix 12 Software version: The software version in ZEN Help About changes to 14.0.12.201
Information about the software ZEN 2012 SP5 black edition Hotfix 12 Software name: ZEN 2012 Service Pack 5 black edition Hotfix 12 Software version: The software version in ZEN Help About changes to 14.0.12.201
Leica TCS SP8 Quick Start Guide
 Leica TCS SP8 Quick Start Guide Leica TCS SP8 System Overview Start-Up Procedure 1. Turn on the CTR Control Box, Fluorescent Light for the microscope stand. 2. Turn on the Scanner Power (1) on the front
Leica TCS SP8 Quick Start Guide Leica TCS SP8 System Overview Start-Up Procedure 1. Turn on the CTR Control Box, Fluorescent Light for the microscope stand. 2. Turn on the Scanner Power (1) on the front
Nature Methods: doi: /nmeth Supplementary Figure 1. Schematic of 2P-ISIM AO optical setup.
 Supplementary Figure 1 Schematic of 2P-ISIM AO optical setup. Excitation from a femtosecond laser is passed through intensity control and shuttering optics (1/2 λ wave plate, polarizing beam splitting
Supplementary Figure 1 Schematic of 2P-ISIM AO optical setup. Excitation from a femtosecond laser is passed through intensity control and shuttering optics (1/2 λ wave plate, polarizing beam splitting
LSM 710 NLO and LSM 780 NLO
 M i c r o s c o p y f r o m C a r l Z e i s s LSM 710 NLO and LSM 780 NLO Information in Depth Innovative Systems for Multiphoton Microscopy Providing Support for Progress and Innovation Biomedical sciences
M i c r o s c o p y f r o m C a r l Z e i s s LSM 710 NLO and LSM 780 NLO Information in Depth Innovative Systems for Multiphoton Microscopy Providing Support for Progress and Innovation Biomedical sciences
Microscopy from Carl Zeiss LSM 710. The Power of Sensitivity. A New Dimension in Confocal Laser Scanning Microscopy
 Microscopy from Carl Zeiss LSM 710 The Power of Sensitivity A New Dimension in Confocal Laser Scanning Microscopy Providing Support for Progress and Innovation The biomedical sciences are considered some
Microscopy from Carl Zeiss LSM 710 The Power of Sensitivity A New Dimension in Confocal Laser Scanning Microscopy Providing Support for Progress and Innovation The biomedical sciences are considered some
1 Co Localization and Working flow with the lsm700
 1 Co Localization and Working flow with the lsm700 Samples -1 slide = mousse intestine, Dapi / Ki 67 with Cy3/ BrDU with alexa 488. -1 slide = mousse intestine, Dapi / Ki 67 with Cy3/ no BrDU (but with
1 Co Localization and Working flow with the lsm700 Samples -1 slide = mousse intestine, Dapi / Ki 67 with Cy3/ BrDU with alexa 488. -1 slide = mousse intestine, Dapi / Ki 67 with Cy3/ no BrDU (but with
Things to check before start-up.
 Byeong Cha Page 1 11/24/2009 Manual for Leica SP2 Confocal Microscope Enter you name, the date, the time, and the account number in the user log book. Things to check before start-up. Make sure that your
Byeong Cha Page 1 11/24/2009 Manual for Leica SP2 Confocal Microscope Enter you name, the date, the time, and the account number in the user log book. Things to check before start-up. Make sure that your
Training Guide for Carl Zeiss LSM 7 MP Multiphoton Microscope
 Training Guide for Carl Zeiss LSM 7 MP Multiphoton Microscope ZEN 2009 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Turn Chameleon TiS laser key from Standby
Training Guide for Carl Zeiss LSM 7 MP Multiphoton Microscope ZEN 2009 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Turn Chameleon TiS laser key from Standby
Basics of confocal imaging (part I)
 Basics of confocal imaging (part I) Swiss Institute of Technology (EPFL) Faculty of Life Sciences Head of BIOIMAGING AND OPTICS BIOP arne.seitz@epfl.ch Lateral resolution BioImaging &Optics Platform Light
Basics of confocal imaging (part I) Swiss Institute of Technology (EPFL) Faculty of Life Sciences Head of BIOIMAGING AND OPTICS BIOP arne.seitz@epfl.ch Lateral resolution BioImaging &Optics Platform Light
The DCS-120 Confocal Scanning FLIM System
 he DCS-120 Confocal Scanning FLIM System he bh DCS-120 confocal scanning FLIM system converts a conventional microscope into a high-performance fluorescence lifetime imaging system. he system is based
he DCS-120 Confocal Scanning FLIM System he bh DCS-120 confocal scanning FLIM system converts a conventional microscope into a high-performance fluorescence lifetime imaging system. he system is based
Contents. Introduction
 Contents Page Contents... 1 Introduction... 1 Starting the System... 2 Introduction to ZEN Efficient Navigation... 5 Setting up the microscope... 10 Configuring the beam path and lasers... 12 Scanning
Contents Page Contents... 1 Introduction... 1 Starting the System... 2 Introduction to ZEN Efficient Navigation... 5 Setting up the microscope... 10 Configuring the beam path and lasers... 12 Scanning
Operation Guide for the Leica SP2 Confocal Microscope Bio-Imaging Facility Hunter College October 2009
 Operation Guide for the Leica SP2 Confocal Microscope Bio-Imaging Facility Hunter College October 2009 Introduction of Fluoresence Confocal Microscopy The first confocal microscope was invented by Princeton
Operation Guide for the Leica SP2 Confocal Microscope Bio-Imaging Facility Hunter College October 2009 Introduction of Fluoresence Confocal Microscopy The first confocal microscope was invented by Princeton
Training Guide for Carl Zeiss LSM 5 LIVE Confocal Microscope
 Training Guide for Carl Zeiss LSM 5 LIVE Confocal Microscope AIM 4.2 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Verify that main power switches on the
Training Guide for Carl Zeiss LSM 5 LIVE Confocal Microscope AIM 4.2 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Verify that main power switches on the
Life Science Instrumentation. New Generation. Light Sheet Fluorescence Microscope. Alph
 Life Science Instrumentation Light Sheet Fluorescence Microscope New Generation Alph Modular Light Sheet Microscope Alpha 3 is a new generation of light sheet fluorescence microscope addressing the needs
Life Science Instrumentation Light Sheet Fluorescence Microscope New Generation Alph Modular Light Sheet Microscope Alpha 3 is a new generation of light sheet fluorescence microscope addressing the needs
TRAINING MANUAL. Olympus FV1000
 TRAINING MANUAL Olympus FV1000 September 2014 TABLE OF CONTENTS A. Start-Up Procedure... 1 B. Visual Observation under the Microscope... 1 C. Image Acquisition... 4 A brief Overview of the Settings...
TRAINING MANUAL Olympus FV1000 September 2014 TABLE OF CONTENTS A. Start-Up Procedure... 1 B. Visual Observation under the Microscope... 1 C. Image Acquisition... 4 A brief Overview of the Settings...
Travel to New Dimensions- LSM 880. The Resolution of a Microscope is limited. The Resolution of a Microscope is limited. Image. Image. Object.
 Travel to New Dimensions- LSM 880 LSM 880: The Power of Sensitivity Our Latest Member of the LSM 880 with GaAsP Detectors Sensitivity, and Ease of Use Innovative High-End Laser Scanning Microscopes from
Travel to New Dimensions- LSM 880 LSM 880: The Power of Sensitivity Our Latest Member of the LSM 880 with GaAsP Detectors Sensitivity, and Ease of Use Innovative High-End Laser Scanning Microscopes from
Guide to Confocal 5. Starting session
 Guide to Confocal 5 Remember that when booking and before starting session you can check for any problems at https://www.bris.ac.uk/biochemistry/uobonly/cif/index.html Starting session Switch on microscope
Guide to Confocal 5 Remember that when booking and before starting session you can check for any problems at https://www.bris.ac.uk/biochemistry/uobonly/cif/index.html Starting session Switch on microscope
Fundamentals of Light Microscopy II: Fluorescence, Deconvolution, Confocal, Multiphoton, Spectral microscopy. Integrated Microscopy Course
 Fundamentals of Light Microscopy II: Fluorescence, Deconvolution, Confocal, Multiphoton, Spectral microscopy Integrated Microscopy Course Review Lecture 1: Microscopy Basics Light train Kohler illumination*
Fundamentals of Light Microscopy II: Fluorescence, Deconvolution, Confocal, Multiphoton, Spectral microscopy Integrated Microscopy Course Review Lecture 1: Microscopy Basics Light train Kohler illumination*
Nikon. King s College London. Imaging Centre. N-SIM guide NIKON IMAGING KING S COLLEGE LONDON
 N-SIM guide NIKON IMAGING CENTRE @ KING S COLLEGE LONDON Starting-up / Shut-down The NSIM hardware is calibrated after system warm-up occurs. It is recommended that you turn-on the system for at least
N-SIM guide NIKON IMAGING CENTRE @ KING S COLLEGE LONDON Starting-up / Shut-down The NSIM hardware is calibrated after system warm-up occurs. It is recommended that you turn-on the system for at least
Confocal Laser Scanning Microscopy
 Name of the Core Facility: Confocal Laser Scanning Microscopy CORE Forschungszentrum Immunologie Mainz Welcome to the CSLM Core Facility: The CLSM Core Facility enables working groups to incorporate high
Name of the Core Facility: Confocal Laser Scanning Microscopy CORE Forschungszentrum Immunologie Mainz Welcome to the CSLM Core Facility: The CLSM Core Facility enables working groups to incorporate high
ZEISS LSM 710 NLO Multiphoton microscope Manual/Quick guide
 ZEISS LSM 710 NLO Multiphoton microscope Manual/Quick guide Matyas Molnar, Biovis 2016 Starting the microscpe 1. Check the microscope if everything looks clean and normal. If not, report it in the logbook.
ZEISS LSM 710 NLO Multiphoton microscope Manual/Quick guide Matyas Molnar, Biovis 2016 Starting the microscpe 1. Check the microscope if everything looks clean and normal. If not, report it in the logbook.
Opterra. Multipoint Scanning Confocal Microscope. Innovation with Integrity. Cell-Friendly, High-Speed, High-Resolution Imaging
 Opterra Multipoint Scanning Confocal Microscope Cell-Friendly, High-Speed, High-Resolution Imaging Innovation with Integrity Fluorescence Microscopy Opterra Multipoint Scanning Confocal Microscope Superior
Opterra Multipoint Scanning Confocal Microscope Cell-Friendly, High-Speed, High-Resolution Imaging Innovation with Integrity Fluorescence Microscopy Opterra Multipoint Scanning Confocal Microscope Superior
Dynamic Confocal Imaging of Living Brain. Advantages and risks of multiphoton microscopy in physiology
 Dynamic Confocal Imaging of Living Brain Advantages and risks of multiphoton microscopy in physiology Confocal laser scanning microscopy In conventional optical microscopy focused and out-offocus light
Dynamic Confocal Imaging of Living Brain Advantages and risks of multiphoton microscopy in physiology Confocal laser scanning microscopy In conventional optical microscopy focused and out-offocus light
Leica_Dye_Finder :53 Uhr Seite 6 Dye Finder LAS AF
 Dye Finder LAS AF Dye Finder Multicolor live cell fluorescence microscopy is limited by the availability of spectrally separable fluorescent dyes. Fluorescent dyes (or spectral GFP variants) with incongruent
Dye Finder LAS AF Dye Finder Multicolor live cell fluorescence microscopy is limited by the availability of spectrally separable fluorescent dyes. Fluorescent dyes (or spectral GFP variants) with incongruent
1.The Problem LIGHT-LEVEL LEVEL IMAGING. light-level level Cameras. 3. Solutions. 2. Low-light LOW-LIGHT
 LOW-LIGHT LIGHT-LEVEL LEVEL IMAGING 1.The Problem 2. Low-light light-level level Cameras 3. Solutions How Much Light? I. Illumination system: 75 W Xenon Arc (~1mW/nm in visible) 490/10 nm exciter filter
LOW-LIGHT LIGHT-LEVEL LEVEL IMAGING 1.The Problem 2. Low-light light-level level Cameras 3. Solutions How Much Light? I. Illumination system: 75 W Xenon Arc (~1mW/nm in visible) 490/10 nm exciter filter
3D light microscopy techniques
 3D light microscopy techniques The image of a point is a 3D feature In-focus image Out-of-focus image The image of a point is not a point Point Spread Function (PSF) 1D imaging 1 1 2! NA = 0.5! NA 2D imaging
3D light microscopy techniques The image of a point is a 3D feature In-focus image Out-of-focus image The image of a point is not a point Point Spread Function (PSF) 1D imaging 1 1 2! NA = 0.5! NA 2D imaging
User Guide to the IBIF Leica TCS SP8 MP Confocal Microscope
 User Guide to the IBIF Leica TCS SP8 MP Confocal Microscope This version: 7.24.14. Introduction The IBIF confocal microscope is made available on a fee-for-use-hour basis to all users who have been trained.
User Guide to the IBIF Leica TCS SP8 MP Confocal Microscope This version: 7.24.14. Introduction The IBIF confocal microscope is made available on a fee-for-use-hour basis to all users who have been trained.
Zeiss LSM 510 Multiphoton Confocal Microscope
 Zeiss LSM 510 Multiphoton Confocal Microscope Quick Start User Guide LSU Health Sciences Research Core Facility Table of Contents 1 Safety... Page 3 2 Turn On the System... Page 4 3 Start Up the ZEN Software.
Zeiss LSM 510 Multiphoton Confocal Microscope Quick Start User Guide LSU Health Sciences Research Core Facility Table of Contents 1 Safety... Page 3 2 Turn On the System... Page 4 3 Start Up the ZEN Software.
OPERATING INSTRUCTIONS
 Zeiss LSM 510 M eta Confocal M icroscope OPERATING INSTRUCTIONS Starting the System: 1. Turn the black knob on the laser box one-quarter turn from Off to On. You will hear the laser cooling mechanisms
Zeiss LSM 510 M eta Confocal M icroscope OPERATING INSTRUCTIONS Starting the System: 1. Turn the black knob on the laser box one-quarter turn from Off to On. You will hear the laser cooling mechanisms
Light Microscopy. Upon completion of this lecture, the student should be able to:
 Light Light microscopy is based on the interaction of light and tissue components and can be used to study tissue features. Upon completion of this lecture, the student should be able to: 1- Explain the
Light Light microscopy is based on the interaction of light and tissue components and can be used to study tissue features. Upon completion of this lecture, the student should be able to: 1- Explain the
Zeiss LSM 510 Confocor III Training Notes. Center for Cell Analysis & Modeling
 Zeiss LSM 510 Confocor III Training Notes Center for Cell Analysis & Modeling Confocor 3 Start Up Go to System Module Turn on Main Switch, System/ PC, and Components Switches Do you need the arc lamp?
Zeiss LSM 510 Confocor III Training Notes Center for Cell Analysis & Modeling Confocor 3 Start Up Go to System Module Turn on Main Switch, System/ PC, and Components Switches Do you need the arc lamp?
ANSWER KEY Lab 2 (IGB): Bright Field and Fluorescence Optical Microscopy and Sectioning
 Phys598BP Spring 2016 University of Illinois at Urbana-Champaign ANSWER KEY Lab 2 (IGB): Bright Field and Fluorescence Optical Microscopy and Sectioning Location: IGB Core Microscopy Facility Microscope:
Phys598BP Spring 2016 University of Illinois at Urbana-Champaign ANSWER KEY Lab 2 (IGB): Bright Field and Fluorescence Optical Microscopy and Sectioning Location: IGB Core Microscopy Facility Microscope:
Leica Sp5 II Confocal User Guide
 Leica Sp5 II Confocal User Guide Turning on the Confocal System (instructions are posted in the room) 1. Turn on Laser Power Button 2. Turn Key to On position 3. Turn on Scanner Power Button 4. Turn on
Leica Sp5 II Confocal User Guide Turning on the Confocal System (instructions are posted in the room) 1. Turn on Laser Power Button 2. Turn Key to On position 3. Turn on Scanner Power Button 4. Turn on
AxioCam HR Success Through Performance
 Microscopy from Carl Zeiss AxioCam HR Success Through Performance The high-resolution camera for digital documentation Superior performance for research and routine work brilliant quality documentation
Microscopy from Carl Zeiss AxioCam HR Success Through Performance The high-resolution camera for digital documentation Superior performance for research and routine work brilliant quality documentation
Invitation for a walk through microscopy. Sebastian Schuchmann Jörg Rösner
 Invitation for a walk through microscopy Sebastian Schuchmann Jörg Rösner joerg.roesner@charite.de Techniques in microscopy Conventional (light) microscopy bright & dark field, phase & interference contrast
Invitation for a walk through microscopy Sebastian Schuchmann Jörg Rösner joerg.roesner@charite.de Techniques in microscopy Conventional (light) microscopy bright & dark field, phase & interference contrast
Supplemental Figure 1: Histogram of 63x Objective Lens z axis Calculated Resolutions. Results from the MetroloJ z axis fits for 5 beads from each
 Supplemental Figure 1: Histogram of 63x Objective Lens z axis Calculated Resolutions. Results from the MetroloJ z axis fits for 5 beads from each lens with a 1 Airy unit pinhole setting. Many water lenses
Supplemental Figure 1: Histogram of 63x Objective Lens z axis Calculated Resolutions. Results from the MetroloJ z axis fits for 5 beads from each lens with a 1 Airy unit pinhole setting. Many water lenses
BASICS OF CONFOCAL IMAGING (PART I)
 BASICS OF CONFOCAL IMAGING (PART I) INTERNAL COURSE 2012 LIGHT MICROSCOPY Lateral resolution Transmission Fluorescence d min 1.22 NA obj NA cond 0 0 rairy 0.61 NAobj Ernst Abbe Lord Rayleigh Depth of field
BASICS OF CONFOCAL IMAGING (PART I) INTERNAL COURSE 2012 LIGHT MICROSCOPY Lateral resolution Transmission Fluorescence d min 1.22 NA obj NA cond 0 0 rairy 0.61 NAobj Ernst Abbe Lord Rayleigh Depth of field
LSM 710 Confocal Microscope Standard Operation Protocol
 LSM 710 Confocal Microscope Standard Operation Protocol Basic Operation Turning on the system 1. Switch on Main power switch 2. Switch on System / PC power button 3. Switch on Components power button 4.
LSM 710 Confocal Microscope Standard Operation Protocol Basic Operation Turning on the system 1. Switch on Main power switch 2. Switch on System / PC power button 3. Switch on Components power button 4.
LSM 780 Confocal Microscope Standard Operation Protocol
 LSM 780 Confocal Microscope Standard Operation Protocol Basic Operation Turning on the system 1. Sign on log sheet according to Actual start time 2. Check Compressed Air supply for the air table 3. Switch
LSM 780 Confocal Microscope Standard Operation Protocol Basic Operation Turning on the system 1. Sign on log sheet according to Actual start time 2. Check Compressed Air supply for the air table 3. Switch
Quick Start Guide. Leica SP5 X
 Quick Start Guide Leica SP5 X Please note: Some of the information in this guide was taken from Leica Microsystems Leica TCS SP5 LAS AF Guide for New Users. This work is licensed under the Creative Commons
Quick Start Guide Leica SP5 X Please note: Some of the information in this guide was taken from Leica Microsystems Leica TCS SP5 LAS AF Guide for New Users. This work is licensed under the Creative Commons
Zeiss LSM 510 Multiphoton Confocal Microscope
 Zeiss LSM 510 Multiphoton Confocal Microscope User Guide LSU Health Sciences Center-Shreveport Research Core Facility Table of Contents 1 Safety... Page 3 2 Turn On the System... Page 4 3 Start Up the
Zeiss LSM 510 Multiphoton Confocal Microscope User Guide LSU Health Sciences Center-Shreveport Research Core Facility Table of Contents 1 Safety... Page 3 2 Turn On the System... Page 4 3 Start Up the
ZEISS LSM 710 CONFOCAL MICROSCOPE USER MANUAL
 ZEISS LSM 710 CONFOCAL MICROSCOPE USER MANUAL START THE SYSTEM... 2 START ZEN SOFTWARE... 3 SET THE TEMPERATURE AND THE CO2 CONTROLLERS... OBSERVATION AT OCULARS... 5 STATIF PRESENTATION... 6 ACQUIRE ONE
ZEISS LSM 710 CONFOCAL MICROSCOPE USER MANUAL START THE SYSTEM... 2 START ZEN SOFTWARE... 3 SET THE TEMPERATURE AND THE CO2 CONTROLLERS... OBSERVATION AT OCULARS... 5 STATIF PRESENTATION... 6 ACQUIRE ONE
PZ-FLIM-110. Piezo Scanning FLIM System. Based on bh s Megapixel FLIM Technology. Complete FLIM Microscopes FLIM Upgrades for Existing Microscopes
 Based on bh s Megapixel FLIM Technology Complete FLIM Microscopes FLIM Upgrades for Existing Microscopes Multidimensional TCSPC technique Sample Scanning by Piezo Stage Compact Electronics, Controlled
Based on bh s Megapixel FLIM Technology Complete FLIM Microscopes FLIM Upgrades for Existing Microscopes Multidimensional TCSPC technique Sample Scanning by Piezo Stage Compact Electronics, Controlled
LEICA TCS SP5 AOBS TANDEM USER MANUAL
 LEICA TCS SP5 AOBS TANDEM USER MANUAL STARTING THE SYSTEM...2 THE LAS AF SOFTWARE...3 THE «ACQUIRE» MENU...5 CHOOSE AND CREATE A SETTING...6 THE CONTROL PANEL...8 THE DMI6000B MICROSCOPE...10 ACQUIRE ONE
LEICA TCS SP5 AOBS TANDEM USER MANUAL STARTING THE SYSTEM...2 THE LAS AF SOFTWARE...3 THE «ACQUIRE» MENU...5 CHOOSE AND CREATE A SETTING...6 THE CONTROL PANEL...8 THE DMI6000B MICROSCOPE...10 ACQUIRE ONE
Fast Laser Raman Microscope RAMAN
 Fast Laser Raman Microscope RAMAN - 11 www.nanophoton.jp Fast Raman Imaging A New Generation of Raman Microscope RAMAN-11 developed by Nanophoton was created by combining confocal laser microscope technology
Fast Laser Raman Microscope RAMAN - 11 www.nanophoton.jp Fast Raman Imaging A New Generation of Raman Microscope RAMAN-11 developed by Nanophoton was created by combining confocal laser microscope technology
Bi/BE 227 Winter Assignment #3. Adding the third dimension: 3D Confocal Imaging
 Bi/BE 227 Winter 2016 Assignment #3 Adding the third dimension: 3D Confocal Imaging Schedule: Jan 20: Assignment Jan 20-Feb 8: Work on assignment Feb 10: Student PowerPoint presentations. Goals for this
Bi/BE 227 Winter 2016 Assignment #3 Adding the third dimension: 3D Confocal Imaging Schedule: Jan 20: Assignment Jan 20-Feb 8: Work on assignment Feb 10: Student PowerPoint presentations. Goals for this
An 8-Channel Parallel Multispectral TCSPC FLIM System
 An 8-Channel Parallel Multispectral TCSPC FLIM System Abstract. We describe a TCSPC FLIM system that uses 8 parallel TCSPC channels to record FLIM data at a peak count rate on the order of 50 10 6 s -1.
An 8-Channel Parallel Multispectral TCSPC FLIM System Abstract. We describe a TCSPC FLIM system that uses 8 parallel TCSPC channels to record FLIM data at a peak count rate on the order of 50 10 6 s -1.
Confocal Application Letter No. 13. Sequential Scan for Leica TCS NT/SP systems
 Confocal Application Letter No. 13 Sequential Scan for Leica TCS NT/SP systems Leica Microsystems Heidelberg GmbH Im Neuenheimer Feld 518 D-69120 Heidelberg Telephone +49 6221 4148 0 Fax +49 6221 414833
Confocal Application Letter No. 13 Sequential Scan for Leica TCS NT/SP systems Leica Microsystems Heidelberg GmbH Im Neuenheimer Feld 518 D-69120 Heidelberg Telephone +49 6221 4148 0 Fax +49 6221 414833
Megapixel FLIM with bh TCSPC Modules
 Megapixel FLIM with bh TCSPC Modules The New SPCM 64-bit Software Abstract: Becker & Hickl have recently introduced version 9.60 of their SPCM TCSPC data acquisition software. SPCM version 9.60 not only
Megapixel FLIM with bh TCSPC Modules The New SPCM 64-bit Software Abstract: Becker & Hickl have recently introduced version 9.60 of their SPCM TCSPC data acquisition software. SPCM version 9.60 not only
Confocal Microscopy. (Increasing contrast and resolu6on using op6cal sec6oning) Lecture 7. November 2017
 Confocal Microscopy (Increasing contrast and resolu6on using op6cal sec6oning) Lecture 7 November 2017 3 Flavours of Microscope Confocal Laser Scanning Problem: Out of Focus Light Spinning disc 2-Photon
Confocal Microscopy (Increasing contrast and resolu6on using op6cal sec6oning) Lecture 7 November 2017 3 Flavours of Microscope Confocal Laser Scanning Problem: Out of Focus Light Spinning disc 2-Photon
Last updated: May 2014 Y.DeGraaf
 FLINDERS MICROSCOPY BIOMEDICAL SERVICES AVAILABLE MICROSCOPES AND SPECIFICATIONS & INFORMATION REGARDING TRAINING FOR NEW USERS Last updated: May 2014 Y.DeGraaf If you have new staff or students (Honours/Masters
FLINDERS MICROSCOPY BIOMEDICAL SERVICES AVAILABLE MICROSCOPES AND SPECIFICATIONS & INFORMATION REGARDING TRAINING FOR NEW USERS Last updated: May 2014 Y.DeGraaf If you have new staff or students (Honours/Masters
Nikon AZ100. Laser Scanning Macro Confocal Microscope. Jordan Briscoe Adam Fries Kyle Marchuk Kaitlin Corbin. May 2017.
 Nikon AZ100 Laser Scanning Macro Confocal Microscope Jordan Briscoe Adam Fries Kyle Marchuk Kaitlin Corbin May 2017 Contents 1 Introduction 2 2 Hardware - Startup 2 3 Software/Operation 4 3.1 Multidimensional
Nikon AZ100 Laser Scanning Macro Confocal Microscope Jordan Briscoe Adam Fries Kyle Marchuk Kaitlin Corbin May 2017 Contents 1 Introduction 2 2 Hardware - Startup 2 3 Software/Operation 4 3.1 Multidimensional
High-sensitivity. optical molecular imaging and high-resolution digital X-ray. In-Vivo Imaging Systems
 High-sensitivity optical molecular imaging and high-resolution digital X-ray In-Vivo Imaging Systems In vivo imaging solutions available in several packages Carestream Molecular Imaging offers a selection
High-sensitivity optical molecular imaging and high-resolution digital X-ray In-Vivo Imaging Systems In vivo imaging solutions available in several packages Carestream Molecular Imaging offers a selection
Confocal imaging on the Leica TCS SP8. 1) Turn the system on. 2) Use TCS user account. 3) Start LAS X software:
 Confocal imaging on the Leica TCS SP8 1) Turn the system on. 2) Use TCS user account. 3) Start LAS X software: 4) Do not touch the microscope while the software is initializing. Choose your options: Turn
Confocal imaging on the Leica TCS SP8 1) Turn the system on. 2) Use TCS user account. 3) Start LAS X software: 4) Do not touch the microscope while the software is initializing. Choose your options: Turn
CONFIGURING. Your Spectroscopy System For PEAK PERFORMANCE. A guide to selecting the best Spectrometers, Sources, and Detectors for your application
 CONFIGURING Your Spectroscopy System For PEAK PERFORMANCE A guide to selecting the best Spectrometers, s, and s for your application Spectral Measurement System Spectral Measurement System Spectrograph
CONFIGURING Your Spectroscopy System For PEAK PERFORMANCE A guide to selecting the best Spectrometers, s, and s for your application Spectral Measurement System Spectral Measurement System Spectrograph
Axio Zoom.V16 The Fluorescence Zoom Microscope for Large Fields
 Product Information Interactive PDF internet-link video/animation Release 1.0 It s About Brilliance. Because Only the Best Is Good Enough In Brief The Advantages The Applications In 1994, the molecular
Product Information Interactive PDF internet-link video/animation Release 1.0 It s About Brilliance. Because Only the Best Is Good Enough In Brief The Advantages The Applications In 1994, the molecular
Akinori Mitani and Geoff Weiner BGGN 266 Spring 2013 Non-linear optics final report. Introduction and Background
 Akinori Mitani and Geoff Weiner BGGN 266 Spring 2013 Non-linear optics final report Introduction and Background Two-photon microscopy is a type of fluorescence microscopy using two-photon excitation. It
Akinori Mitani and Geoff Weiner BGGN 266 Spring 2013 Non-linear optics final report Introduction and Background Two-photon microscopy is a type of fluorescence microscopy using two-photon excitation. It
Bio 407. Applied microscopy. Introduction into light microscopy. José María Mateos. Center for Microscopy and Image Analysis
 Center for Microscopy and Image Analysis Bio 407 Applied Introduction into light José María Mateos Fundamentals of light Compound microscope Microscope composed of an objective and an additional lens (eyepiece,
Center for Microscopy and Image Analysis Bio 407 Applied Introduction into light José María Mateos Fundamentals of light Compound microscope Microscope composed of an objective and an additional lens (eyepiece,
Training Guide for Carl Zeiss LSM 880 with AiryScan FAST
 Training Guide for Carl Zeiss LSM 880 with AiryScan FAST ZEN 2.3 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2018) Power ON Routine 1 2 Turn ON Main Switch from the remote control
Training Guide for Carl Zeiss LSM 880 with AiryScan FAST ZEN 2.3 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2018) Power ON Routine 1 2 Turn ON Main Switch from the remote control
Imaging Retreat - UMASS Customized real-time confocal and 2-photon imaging
 Imaging Retreat - UMASS 2012 Customized real-time confocal and 2-photon imaging Mike Sanderson Department of Microbiology and Physiological Systems University of Massachusetts Medical School Thanks for
Imaging Retreat - UMASS 2012 Customized real-time confocal and 2-photon imaging Mike Sanderson Department of Microbiology and Physiological Systems University of Massachusetts Medical School Thanks for
3D light microscopy techniques
 3D light microscopy techniques The image of a point is a 3D feature In-focus image Out-of-focus image The image of a point is not a point Point Spread Function (PSF) 1D imaging 2D imaging 3D imaging Resolution
3D light microscopy techniques The image of a point is a 3D feature In-focus image Out-of-focus image The image of a point is not a point Point Spread Function (PSF) 1D imaging 2D imaging 3D imaging Resolution
Leica SP8 TCS Users Manual
 Version : 07/08/0 Leica SP8 TCS Users Manual Start up:. Turn the PC Microscope, Scanner Power, Laser Power, and the Laser Emission key to on (bottom right of desk).. Turn on the fluorescent lamp (top left
Version : 07/08/0 Leica SP8 TCS Users Manual Start up:. Turn the PC Microscope, Scanner Power, Laser Power, and the Laser Emission key to on (bottom right of desk).. Turn on the fluorescent lamp (top left
IC 2 S High Performance Objectives
 M i c r o s c o p y f r o m C a r l Z e i s s IC 2 S igh Performance Objectives for Biomedical Applications with Laser Based Imaging Systems LSM,, ConfoCor, TIRF and ELYRA Carl Zeiss offers a large range
M i c r o s c o p y f r o m C a r l Z e i s s IC 2 S igh Performance Objectives for Biomedical Applications with Laser Based Imaging Systems LSM,, ConfoCor, TIRF and ELYRA Carl Zeiss offers a large range
Fast Laser Raman Microscope RAMAN
 Fast Laser Raman Microscope RAMAN - 11 www.nanophoton.jp Fast Raman Imaging A New Generation of Raman Microscope RAMAN-11 developed by Nanophoton was created by combining confocal laser microscope technology
Fast Laser Raman Microscope RAMAN - 11 www.nanophoton.jp Fast Raman Imaging A New Generation of Raman Microscope RAMAN-11 developed by Nanophoton was created by combining confocal laser microscope technology
picoemerald Tunable Two-Color ps Light Source Microscopy & Spectroscopy CARS SRS
 picoemerald Tunable Two-Color ps Light Source Microscopy & Spectroscopy CARS SRS 1 picoemerald Two Colors in One Box Microscopy and Spectroscopy with a Tunable Two-Color Source CARS and SRS microscopy
picoemerald Tunable Two-Color ps Light Source Microscopy & Spectroscopy CARS SRS 1 picoemerald Two Colors in One Box Microscopy and Spectroscopy with a Tunable Two-Color Source CARS and SRS microscopy
Nikon C1si Spectral Laser Scanning Confocal Microscope. User Guide
 Nikon C1si Spectral Laser Scanning Confocal Microscope User Guide Contents: C1Si Turn-On/ShutDown Procedures... 2 Overview... 4 Setup for epi-illumination to view through the eyepieces:... 5 Setup for
Nikon C1si Spectral Laser Scanning Confocal Microscope User Guide Contents: C1Si Turn-On/ShutDown Procedures... 2 Overview... 4 Setup for epi-illumination to view through the eyepieces:... 5 Setup for
FEMTOSMART. Benefits. Features
 FEMTOSMART Extremely large space under the objective For in vivo studies Field upgradability Patented imaging technologies Flexible scanning methods Maximal photon collection Elevated, column-based body
FEMTOSMART Extremely large space under the objective For in vivo studies Field upgradability Patented imaging technologies Flexible scanning methods Maximal photon collection Elevated, column-based body
Instructions for the Experiment
 Instructions for the Experiment Excitonic States in Atomically Thin Semiconductors 1. Introduction Alongside with electrical measurements, optical measurements are an indispensable tool for the study of
Instructions for the Experiment Excitonic States in Atomically Thin Semiconductors 1. Introduction Alongside with electrical measurements, optical measurements are an indispensable tool for the study of
Fastest high definition Raman imaging. Fastest Laser Raman Microscope RAMAN
 Fastest high definition Raman imaging Fastest Laser Raman Microscope RAMAN - 11 www.nanophoton.jp Observation A New Generation in Raman Observation RAMAN-11 developed by Nanophoton was newly created by
Fastest high definition Raman imaging Fastest Laser Raman Microscope RAMAN - 11 www.nanophoton.jp Observation A New Generation in Raman Observation RAMAN-11 developed by Nanophoton was newly created by
CONFOCAL MICROSCOPE (Zeiss LSM 510 META v4.2)
 Wellcome Trust Centre for Human Genetics Molecular Cytogenetics and Microscopy Core CONFOCAL MICROSCOPE (Zeiss LSM 510 META v4.2) 1) STARTING THE SYSTEM Abridged INSTRUCTIONS Switch on the mercury bulb
Wellcome Trust Centre for Human Genetics Molecular Cytogenetics and Microscopy Core CONFOCAL MICROSCOPE (Zeiss LSM 510 META v4.2) 1) STARTING THE SYSTEM Abridged INSTRUCTIONS Switch on the mercury bulb
