TRAINING MANUAL. Multiphoton Microscopy LSM 510 META-NLO
|
|
|
- Grace Mills
- 5 years ago
- Views:
Transcription
1 TRAINING MANUAL Multiphoton Microscopy LSM 510 META-NLO September 2010
2 Multiphoton Microscopy Training Manual Multiphoton microscopy is only available on the LSM 510 META-NLO system. This system is equipped with the Chameleon laser, which is a Ti:Sa femtosecond laser. The pre-requisite for the multiphoton training is the Introduction to Confocal Microscopy training. Many of the steps for setting up and acquiring a multiphoton image is the same as for single photon imaging. Therefore this training only covers the steps that differ from the introductory training. Multiphoton Training includes: A. Introduction to Multiphoton Microscopy B. Start-up Procedure C. Turning on the Chameleon Laser D. Setting the Configuration E. Scan Parameters F. Laser Safety G. Shut-down Procedure Please refer to the LSM 510 META Training Manual for a more complete description of all of the steps.
3 A. INTRODUCTION - Multiphoton Microscopy Multiphoton microscopy is another type of laser scanning microscopy that also provides a single plane image, however multiphoton microscopy achieves this without using a pinhole. Instead, only the fluorescent molecules within the plane of focus are excited eliminating the need for the pinhole since there is no excitation and subsequent emission outside the focal plane. Excitation of only the fluorescent molecules in the focal plane is achieved by a femtosecond laser that sends photons of light with lower energy (longer wavelength) in rapid succession. Multiple photons of the lower energy light must be absorbed in order for the fluorochrome to reach the excited state and the focal plane is the only place where there is sufficient number of photons (in both space and time) for this to occur. Single Photon Multiphoton 2. Transition 2. Transition 1. Excitation 3. Emission 1. Excitation 3. Emission 1 photon 2 photons 1 photon with higher energy (Shorter wavelength) is required for excitation 2 photons with lower energy (Longer wavelength) is required for excitation Single Photon Multi Multiphoton Photon Although the wavelengths used for single and multiphoton excitation are different, the emission spectrum is the same. Emission Intensity (%) 100 Single photon excitation Emission Intensity (%) photon excitation wavelength (nm) wavelength (nm) wavelength (nm)
4 A. INTRODUCTION Continued... Advantages of Multiphoton Microscopy: Longer wavelengths (Red or Near-Infrared light) Longer wavelength light is less biologically damaging Excitation is limited to the focal plane The only place where there is sufficient number of photons (in both space and time) is the focal plane therefore very little, if any, out-of-focus fluorescence occurs ( therefore a pinhole is not required) Photon Specimen Excitation is restricted to the focal plane due to photon crowding (In both space and time) Since excitation is limited to the focal plane, photobleaching is also limited to the focal plane Single Photon Excitation Multiphoton Excitation Coverslip } FOCAL PLANE Slide Photobleached Fluorochrome Photobleaching is limited to the focal plane with multiphoton excitation
5 A. INTRODUCTION Continued... Advantages of Multiphoton Microscopy Continued... Image thicker specimens Light Scatters Both single and multi-photon excitation and resulting fluorescence can be scattered in the specimen The extent of scattering depends on the specimen Generally the laser power is increased to compensate for scatter } FOCAL PLANE In both single and multi-photon microscopy the excitation laser is scattered in thick specimens. The laser intensity is increased to compensate for this scatter. Single Photon Multiphoton Emission in multiple planes with single photon excitation { FOCAL Out-of-focus emission scattered and passes through the pinhole to the detector FOCAL PLANE quality (contrast) of the image by collecting out-of focus fluorescence PLANE In-focus emission scattered and blocked by the pinhole in emission collected from the focal plane In-focus emission passed through the pinhole and is collected Excitation only occurs in the focal plane because the absorption of 2 photons at the same time only happens in the focal plane. Therefore, emission only originates from the focal plane Since there is no pinhole in multiphoton microscopy scattered emission (along with nonscattered) is able to reach the detector Detector Detector
6 A. INTRODUCTION Continued... Some Disadvantages or Limitations of Multiphoton Microscopy Less lateral and axial resolution When light from the various points of a specimen passes through the objective and is reconstituted as an image, the various points of the specimen appear in the image as small patterns (not points) known as Airy patterns. This is caused by diffraction or scattering of the light as it passes through the specimen and the circular back aperture of the objective Airy Pattern Resolution the minimum detectable distance between two closely space specimen points the limit at which 2 airy disks can be resolved into separate entities (Rayleigh Criterion) These points can be resolved 3 critical characteristics set the resolution limit of the microscope These 2 points cannot be resolved as separate points NA at a fixed, size of airy disk, resolution Numerical Aperture: the light cone size decreases with NA (at a fixed ) and produces a corresponding decrease in the size of the Airy disks ( resolution) Refractive Index: numerical aperture can be dramatically increased by immersion medium such as oil, glycerin or water Wavelength: an increase in the illumination wavelength at a fixed NA will result in an increase in Airy pattern ( resolution) multiphoton microscopy uses longer excitation compared to single photon (therefore multiphoton microscopy has resolution) Increasing illumination Increasing NA illumination size of airy disk resolution
7 A. INTRODUCTION Continued... Some Disadvantages or Limitations of Multiphoton Microscopy Continued... Excitation spectra difficult to predict and measure few sources of multiphoton excitation information available twice the wavelength of the single photon excitation may not necessarily be the most efficient for multiphoton excitation Single Photon Excitation Multiphoton Excitation FITC Rhodamine Absorption Wavelength Thermal damage to specimen Water molecules absorb more energy from longer wavelengths of light (NIR and IR) compared to shorter wavelengths (VIS and UV) Heat is generated as water molecules absorb the energy from NIR and IR light. Since the excitation wavelength in multiphoton microscopy is in the NIR and IR range, thermal damage can be a problem as water molecules (both intracellular and Extracellular) in the sample absorb the excitation light generating heat
8 B. START-UP PROCEDURE - Multiphoton Microscopy 1. If the chiller is in Standby Mode, press the Standby/Run button to switch the chiller from o Standby Mode to Run Mode. (The chiller is set to ~25 C and the current temperature will be displayed.) NOTE: DO NOT OPERATE THE CHAMELEON LASER WITH THE CHILLER IN STANDBY MODE!!!! THE CHILLER MUST ALWAYS BE TURNED ON WHEN USING THE CHAMELEON LASER!!!! 2. Turn on the mercury burner 3. Turn both the PC and Components buttons on the REMOTE CONTROL switch to the ON position 4. Turn on the computer 5. Log into user profile - press Ctrl, Alt and Delete simultaneously - enter Userid and password 6. Double click on the LSM 510 icon start the LSM software 7. In the LSM 510 Switchboard window select Scan New Images and then Start Expert Mode The system will go through an instrument initialization and then the main LSM Toolbar will appear
9 C. TURNING ON CHAMELEON LASER - Multiphoton Microscopy Before turning on the Chameleon laser: make sure the chiller is in Run Mode (not in Standby Mode) THE CHILLER MUST BE ON AT ALL TIMES WHEN THE CHAMELEON LASER IS TURNED ON!!!!! make sure the key on the Chameleon power supply (under the table) is in the On position If the chiller and Chameleon is in Standby Mode, go to step #1 in the Multiphoton Microscopy Start-up Procedure (Section A) 1. click on Chameleon to select the Chameleon laser 2. Click the On button to turn the laser on Mode Locked 3. Click on Modify to open the Laser Modify Control window 4. Enter the excitation wavelength ( nm only) for the Chameleon laser and then click on Store. Wait a few seconds until Mode Lock is re-established Close the laser control window if all of the lasers needed are turned on DO NOT adjust the fine tuning AO-Frequency!!!
10 D. CONFIGURATIONS - Multiphoton Microscopy All of the steps for setting the configurations are the same for both single photon and multiphoton microscopy. There are only a few things to keep in mind. multiphoton can be used in combination with single photon (ie. Argon & HeNe lasers) use as a single or multi-track use Ch S, Ch2 or Ch3 always use a KP (short pass) main dichroic beam splitter always use either a BP or KP emission filter (never use a LP filter) Refer to the General Confocal Microscopy Training Manual for a more detailed description and instruction on the configurations 1. Select Channel Mode 2. Select Single Track or Multi Track 3. Click on Excitation to open the Laser Control Window 4. Activate the excitation wavelengths (check box) and setting of excitation intensities (slider) NEVER SET THE CHAMELEON LASER >10%!!!! * * 5. Click on main dichroic beam splitter (HFT) to open the list of beam splitters and select the appropriate dichroic for the excitation laser line(s) you have activated. The main dichroic beam splitter separates excitation from emission NT 80/20 = no filter * HFT = main dichroic beam splitter KP = short pass (LSM 510 META - NLO only) - use with the Chameleon laser Always use a KP beam splitter with the Chameleon laser
11 D. CONFIGURATIONS Continued Click on the first secondary beam splitter (NFT) to open the list of options for this position. This beam splitter directs the appropriate wavelengths of emission to Channel S and/or Channel 2 and 3. Select the appropriate mirror or filter. None = all emission will pass through to Channel S Mirror = all emission will be directed to Channel 2 and 3 Plate = all emission will pass through to Channel S NFT = secondary dichroic beam splitter KP = short pass filter 7. Click on the second secondary beam splitter to open the list or options for this position. This beam splitter directs the appropriate wavelengths of emission to Channel 2 and/or Channel 3. Select the appropriate mirror or filter. Mirror = all emission will be directed to Channel 2 NFT 490 = all emission with wavelengths less than 490 nm will be directed to Channel 2 and emission with wavelengths greater than 490 nm will pass through to Channel 3 NFT 545 = all emission with wavelengths less than 545 nm will be directed to Channel 2 and emission with wavelengths greater than 545 nm will pass through to Channel 3 BG 39 = all emission will pass through to Channel 3
12 D. CONFIGURATIONS Continued Click on Ch S and select the adjust the sliders or click on the Emission Filter for Channel 2 and Channel 3 to open the list of emission filters available. 9. Select the appropriate emission filter and then check the box to turn on the corresponding channel. Use either a KP or BP filter for multiphoton microscopy. Do not use a LP filter. Band Pass (BP) - permit only wavelengths of emission within the range indicated to pass through to the PMT - Band pass filters can be used with all of the lasers Short Pass (KP) - permit emission with wavelengths below the threshold indicated to pass through to the PMT Channel 2 Emission Filters Channel 3 Emission Filters X X - Short pass filters are used only with the Chameleon laser and not the Argon or HeNe lasers. Long Pass (LP) - Long pass filters permit emission with wavelengths above the threshold indicated to pass through to the PMT - Long pass filters can be used with the Argon and HeNe lasers, but should not be used with the Chameleon laser 10. Click on the colour box to o select the appropriate pseudo-colour for that particular channel. 11. Add another track and repeat steps #1-11 if necessary.
13 E. INITIAL SCAN PARAMETERS Set the initial Scan Parameters the same as for single photon with the exception of setting the pinhole: When using the Chameleon laser click on Max to open the pinhole multiphoton microscopy does not require a pinhole emission only occurs when 2 or more photons hit the fluorophore at the same time this only occurs within the focal spot F. LASER SAFETY Before you put your sample on the stage there are some very important laser safety precautions that need to be taken. But first, a little about the hazards and classification of lasers: Lasers are classified into 4 groups according to the maximum power or energy of the beam, wavelength output, and pulse repetition Generally, the classification indicates the capability of the laser or laser system to produce injury with higher class numbers indicating a greater potential hazard. Class 1 - lasers or laser systems that do not pose a hazard under normal operating conditions Class 2 - low power visible lasers that are incapable of causing eye injury unless viewed directly for extended periods of time Class 3A - lasers or laser systems that have a low risk of injury but normally would not injure the eye unless directly viewed through binoculars or similar optical devices Class 3B - lasers or laser systems that can produce accidental injuries to the eye from viewing the direct beam or a specular reflection, but normally is not a hazard from diffuse reflection (unless viewed through an optical instrument) Class 4 - lasers that have eye and skin hazards from the direct beam, specular reflections and diffuse reflections. This is a very brief description of the lasers classes. Please refer to the UWO Laser Safety Manual for a more detailed description.
14 F. LASER SAFETY Continued... Specifically for our systems, the Argon, HeNe and 405nm Laser Diode are all Class 3 lasers whereas the CHAMELEON LASER IS A CLASS 4 LASER!!!! When enclosed within the microscope system, all of the lasers pose very little risk and are therefore considered Class 1. Regardless, there still very important safety steps that must be followed when using the Chameleon laser. Since the Argon, HeNe and 405nm Laser Diode lasers emit in the visible range, the laser beam can be seen when running. The Chameleon Laser however emits in the near infrared range, THEREFORE YOU CANNOT SEE THE LASER BEAM!! Class 4 lasers can damage your eyes and skin from the direct beam and REFLECTED BEAM!!! NEVER MANIPULATE ADJUST YOUR SLIDE WITH THE LASER ON to avoid potentially reflecting the laser beam into your eyes and/or exposing your skin to the laser beam. There are 3 main steps to take before you do any manipulation of your sample on the stage to ensure the laser is turned off: 1. Click Stop in the Scan Control Window 2. Click Vis in the main toolbar 3. Tilt the light carrier back to disconnect the laser circuit. There are also goggles and shields available for your protection
15 G. SHUT-DOWN PROCEDURE 1. Close all open windows, except the main LSM Toolbar 2. Open the Laser Control box Cool the Argon Laser on Standby highlight the argon laser click on Standby and then Off wait until cooling in the status bar switches to connected IMPORTANT: the argon laser must be cooled before turning the system off Turn the NeNe lasers off highlight the appropriate laser click Off Turn off the Chameleon laser highlight the Chameleon laser click Off turn key on the Chameleon power supply (under the table) to Standby 3. Click on File in the main LSM Toolbar and then Exit. Click Exit in the main switchboard. ( A window will open reminding you the lasers are on and do not turn the system off. Click OK) 4. Log off the computer (ctl + alt + del) and record use in the log sheet. 5. CLEAN THE OBJECTIVE!!!! if not cleaned properly. These objectives are very expensive and are damaged very easily To clean: wipe off any oil on the objective with lens paper use lens paper damp with lens cleaner to clean the objective dry the objective with a clean and dry piece of lens paper IF YOU DO NOT CLEAN THE OBJECTIVES PROPERLY, YOUR MICROSCOPE PRIVILEGES WILL BE TAKEN AWAY.
ZEISS LSM510META confocal manual
 ZEISS LSM510META confocal manual Switching on the system 1) Switch on the Remote Control button located on the table to the right of the microscope. This is the main switch for the whole system including
ZEISS LSM510META confocal manual Switching on the system 1) Switch on the Remote Control button located on the table to the right of the microscope. This is the main switch for the whole system including
Training Guide for Carl Zeiss LSM 510 META Confocal Microscope
 Training Guide for Carl Zeiss LSM 510 META Confocal Microscope AIM 4.2 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Turn ON Components and System/PC switches
Training Guide for Carl Zeiss LSM 510 META Confocal Microscope AIM 4.2 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Turn ON Components and System/PC switches
Microscope Confocal LSM510 META
 Microscope Confocal LSM510 META Welcome to the Zeiss LSM 510 Meta Confocal tutorial. Before using the LSM 510 META, Log off any other computer that is open with your personal login. You will need to put
Microscope Confocal LSM510 META Welcome to the Zeiss LSM 510 Meta Confocal tutorial. Before using the LSM 510 META, Log off any other computer that is open with your personal login. You will need to put
LSM 510 Meta Training Notes
 LSM 510 Meta Training Notes Turning on the system Turn on X-Cite power supply. This supplies light for epifluorescence for viewing your samples through the microscope. Turn on the remote control switch.
LSM 510 Meta Training Notes Turning on the system Turn on X-Cite power supply. This supplies light for epifluorescence for viewing your samples through the microscope. Turn on the remote control switch.
Zeiss LSM 510 Confocor III Training Notes. Center for Cell Analysis & Modeling
 Zeiss LSM 510 Confocor III Training Notes Center for Cell Analysis & Modeling Confocor 3 Start Up Go to System Module Turn on Main Switch, System/ PC, and Components Switches Do you need the arc lamp?
Zeiss LSM 510 Confocor III Training Notes Center for Cell Analysis & Modeling Confocor 3 Start Up Go to System Module Turn on Main Switch, System/ PC, and Components Switches Do you need the arc lamp?
LSM 510 Training Notes
 LSM 510 Training Notes Turning on the system Turn on the arc lamp, found on the bench top left of the microscope. This supplies light for epifluorescence for viewing your samples through the microscope.
LSM 510 Training Notes Turning on the system Turn on the arc lamp, found on the bench top left of the microscope. This supplies light for epifluorescence for viewing your samples through the microscope.
Training Guide for Carl Zeiss LSM 5 LIVE Confocal Microscope
 Training Guide for Carl Zeiss LSM 5 LIVE Confocal Microscope AIM 4.2 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Verify that main power switches on the
Training Guide for Carl Zeiss LSM 5 LIVE Confocal Microscope AIM 4.2 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Verify that main power switches on the
Zeiss 880 Training Notes Zen 2.3
 Zeiss 880 Training Notes Zen 2.3 1 Turn on the HXP 120V Lamp 2 Turn on Main Power Switch Turn on the Systems PC Switch Turn on the Components Switch. 3 4 5 Turn on the PC and log into your account. Start
Zeiss 880 Training Notes Zen 2.3 1 Turn on the HXP 120V Lamp 2 Turn on Main Power Switch Turn on the Systems PC Switch Turn on the Components Switch. 3 4 5 Turn on the PC and log into your account. Start
Things to check before start-up.
 Byeong Cha Page 1 11/24/2009 Manual for Leica SP2 Confocal Microscope Enter you name, the date, the time, and the account number in the user log book. Things to check before start-up. Make sure that your
Byeong Cha Page 1 11/24/2009 Manual for Leica SP2 Confocal Microscope Enter you name, the date, the time, and the account number in the user log book. Things to check before start-up. Make sure that your
Training Guide for Leica SP8 Confocal/Multiphoton Microscope
 Training Guide for Leica SP8 Confocal/Multiphoton Microscope LAS AF v3.3 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Turn ON power switch for epifluorescence
Training Guide for Leica SP8 Confocal/Multiphoton Microscope LAS AF v3.3 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Turn ON power switch for epifluorescence
Maria Smedh, Centre for Cellular Imaging. Maria Smedh, Centre for Cellular Imaging
 Nonlinear microscopy I: Two-photon fluorescence microscopy Multiphoton Microscopy What is multiphoton imaging? Applications Different imaging modes Advantages/disadvantages Scattering of light in thick
Nonlinear microscopy I: Two-photon fluorescence microscopy Multiphoton Microscopy What is multiphoton imaging? Applications Different imaging modes Advantages/disadvantages Scattering of light in thick
Practical work no. 3: Confocal Live Cell Microscopy
 Practical work no. 3: Confocal Live Cell Microscopy Course Instructor: Mikko Liljeström (MIU) 1 Background Confocal microscopy: The main idea behind confocality is that it suppresses the signal outside
Practical work no. 3: Confocal Live Cell Microscopy Course Instructor: Mikko Liljeström (MIU) 1 Background Confocal microscopy: The main idea behind confocality is that it suppresses the signal outside
3D light microscopy techniques
 3D light microscopy techniques The image of a point is a 3D feature In-focus image Out-of-focus image The image of a point is not a point Point Spread Function (PSF) 1D imaging 1 1 2! NA = 0.5! NA 2D imaging
3D light microscopy techniques The image of a point is a 3D feature In-focus image Out-of-focus image The image of a point is not a point Point Spread Function (PSF) 1D imaging 1 1 2! NA = 0.5! NA 2D imaging
Operation Guide for the Leica SP2 Confocal Microscope Bio-Imaging Facility Hunter College October 2009
 Operation Guide for the Leica SP2 Confocal Microscope Bio-Imaging Facility Hunter College October 2009 Introduction of Fluoresence Confocal Microscopy The first confocal microscope was invented by Princeton
Operation Guide for the Leica SP2 Confocal Microscope Bio-Imaging Facility Hunter College October 2009 Introduction of Fluoresence Confocal Microscopy The first confocal microscope was invented by Princeton
Operating Instructions for Zeiss LSM 510
 Operating Instructions for Zeiss LSM 510 Location: GNL 6.312q (BSL3) Questions? Contact: Maxim Ivannikov, maivanni@utmb.edu 1 Attend A Complementary Training Before Using The Microscope All future users
Operating Instructions for Zeiss LSM 510 Location: GNL 6.312q (BSL3) Questions? Contact: Maxim Ivannikov, maivanni@utmb.edu 1 Attend A Complementary Training Before Using The Microscope All future users
SHORT INSTRUCTIONS FOR OPERATING LSM1/2 (Zeiss LSM510) AT CIAN Version 1.4, September 2014
 CIAN LSM1 or LSM2 short instructions, version 1.4, September 2014 page 1 of 6 SHORT INSTRUCTIONS FOR OPERATING LSM1/2 (Zeiss LSM510) AT CIAN Version 1.4, September 2014 Before starting To work with LSM1
CIAN LSM1 or LSM2 short instructions, version 1.4, September 2014 page 1 of 6 SHORT INSTRUCTIONS FOR OPERATING LSM1/2 (Zeiss LSM510) AT CIAN Version 1.4, September 2014 Before starting To work with LSM1
Zeiss 780 Training Notes
 Zeiss 780 Training Notes Turn on Main Switch, System PC and Components Switches 780 Start up sequence Do you need the argon laser (458, 488, 514 nm lines)? Yes Turn on the laser s main power switch and
Zeiss 780 Training Notes Turn on Main Switch, System PC and Components Switches 780 Start up sequence Do you need the argon laser (458, 488, 514 nm lines)? Yes Turn on the laser s main power switch and
LSM 510 META in Chang Gung University
 Content LSM 510 META in Chang ung University LSM 510 META 路 理 The features and applications of LSM 510 META 01-09 Introduction of the hardware 10-12 Fluorescence observation in conventional microscope
Content LSM 510 META in Chang ung University LSM 510 META 路 理 The features and applications of LSM 510 META 01-09 Introduction of the hardware 10-12 Fluorescence observation in conventional microscope
Quick Guide. LSM 5 MP, LSM 510 and LSM 510 META. Laser Scanning Microscopes. We make it visible. M i c r o s c o p y f r o m C a r l Z e i s s
 LSM 5 MP, LSM 510 and LSM 510 META M i c r o s c o p y f r o m C a r l Z e i s s Quick Guide Laser Scanning Microscopes LSM Software ZEN 2007 August 2007 We make it visible. Contents Page Contents... 1
LSM 5 MP, LSM 510 and LSM 510 META M i c r o s c o p y f r o m C a r l Z e i s s Quick Guide Laser Scanning Microscopes LSM Software ZEN 2007 August 2007 We make it visible. Contents Page Contents... 1
Why and How? Daniel Gitler Dept. of Physiology Ben-Gurion University of the Negev. Microscopy course, Michmoret Dec 2005
 Why and How? Daniel Gitler Dept. of Physiology Ben-Gurion University of the Negev Why use confocal microscopy? Principles of the laser scanning confocal microscope. Image resolution. Manipulating the
Why and How? Daniel Gitler Dept. of Physiology Ben-Gurion University of the Negev Why use confocal microscopy? Principles of the laser scanning confocal microscope. Image resolution. Manipulating the
Internal Medicine Imaging Core Emory University Department of Medicine
 Internal Medicine Imaging Core Emory University Department of Medicine 1 OPERATION OF THE ZEISS LSM 510 META YOU MUST SIGN UP TO USE THE MICROSCOPE OR COMPUTER EVERY TIME NO EXCEPTIONS Before attempting
Internal Medicine Imaging Core Emory University Department of Medicine 1 OPERATION OF THE ZEISS LSM 510 META YOU MUST SIGN UP TO USE THE MICROSCOPE OR COMPUTER EVERY TIME NO EXCEPTIONS Before attempting
Zeiss LSM 510 Multiphoton Confocal Microscope
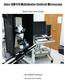 Zeiss LSM 510 Multiphoton Confocal Microscope Quick Start User Guide LSU Health Sciences Research Core Facility Table of Contents 1 Safety... Page 3 2 Turn On the System... Page 4 3 Start Up the ZEN Software.
Zeiss LSM 510 Multiphoton Confocal Microscope Quick Start User Guide LSU Health Sciences Research Core Facility Table of Contents 1 Safety... Page 3 2 Turn On the System... Page 4 3 Start Up the ZEN Software.
Zeiss LSM 510 Multiphoton Confocal Microscope
 Zeiss LSM 510 Multiphoton Confocal Microscope User Guide LSU Health Sciences Center-Shreveport Research Core Facility Table of Contents 1 Safety... Page 3 2 Turn On the System... Page 4 3 Start Up the
Zeiss LSM 510 Multiphoton Confocal Microscope User Guide LSU Health Sciences Center-Shreveport Research Core Facility Table of Contents 1 Safety... Page 3 2 Turn On the System... Page 4 3 Start Up the
Leica SP8 TCS Users Manual
 Version : 07/08/0 Leica SP8 TCS Users Manual Start up:. Turn the PC Microscope, Scanner Power, Laser Power, and the Laser Emission key to on (bottom right of desk).. Turn on the fluorescent lamp (top left
Version : 07/08/0 Leica SP8 TCS Users Manual Start up:. Turn the PC Microscope, Scanner Power, Laser Power, and the Laser Emission key to on (bottom right of desk).. Turn on the fluorescent lamp (top left
LSM 710 Confocal Microscope Standard Operation Protocol
 LSM 710 Confocal Microscope Standard Operation Protocol Basic Operation Turning on the system 1. Switch on Main power switch 2. Switch on System / PC power button 3. Switch on Components power button 4.
LSM 710 Confocal Microscope Standard Operation Protocol Basic Operation Turning on the system 1. Switch on Main power switch 2. Switch on System / PC power button 3. Switch on Components power button 4.
Quick Start Guide. Leica SP5 X
 Quick Start Guide Leica SP5 X Please note: Some of the information in this guide was taken from Leica Microsystems Leica TCS SP5 LAS AF Guide for New Users. This work is licensed under the Creative Commons
Quick Start Guide Leica SP5 X Please note: Some of the information in this guide was taken from Leica Microsystems Leica TCS SP5 LAS AF Guide for New Users. This work is licensed under the Creative Commons
3. are adherent cells (ie. cells in suspension are too far away from the coverslip)
 Before you begin, make sure your sample... 1. is seeded on #1.5 coverglass (thickness = 0.17) 2. is an aqueous solution (ie. fixed samples mounted on a slide will not work - not enough difference in refractive
Before you begin, make sure your sample... 1. is seeded on #1.5 coverglass (thickness = 0.17) 2. is an aqueous solution (ie. fixed samples mounted on a slide will not work - not enough difference in refractive
Bi/BE 227 Winter Assignment #3. Adding the third dimension: 3D Confocal Imaging
 Bi/BE 227 Winter 2016 Assignment #3 Adding the third dimension: 3D Confocal Imaging Schedule: Jan 20: Assignment Jan 20-Feb 8: Work on assignment Feb 10: Student PowerPoint presentations. Goals for this
Bi/BE 227 Winter 2016 Assignment #3 Adding the third dimension: 3D Confocal Imaging Schedule: Jan 20: Assignment Jan 20-Feb 8: Work on assignment Feb 10: Student PowerPoint presentations. Goals for this
OPERATING INSTRUCTIONS
 Zeiss LSM 510 M eta Confocal M icroscope OPERATING INSTRUCTIONS Starting the System: 1. Turn the black knob on the laser box one-quarter turn from Off to On. You will hear the laser cooling mechanisms
Zeiss LSM 510 M eta Confocal M icroscope OPERATING INSTRUCTIONS Starting the System: 1. Turn the black knob on the laser box one-quarter turn from Off to On. You will hear the laser cooling mechanisms
Akinori Mitani and Geoff Weiner BGGN 266 Spring 2013 Non-linear optics final report. Introduction and Background
 Akinori Mitani and Geoff Weiner BGGN 266 Spring 2013 Non-linear optics final report Introduction and Background Two-photon microscopy is a type of fluorescence microscopy using two-photon excitation. It
Akinori Mitani and Geoff Weiner BGGN 266 Spring 2013 Non-linear optics final report Introduction and Background Two-photon microscopy is a type of fluorescence microscopy using two-photon excitation. It
Confocal Microscopy. Kristin Jensen
 Confocal Microscopy Kristin Jensen 17.11.05 References Cell Biological Applications of Confocal Microscopy, Brian Matsumoto, chapter 1 Studying protein dynamics in living cells,, Jennifer Lippincott-Schwartz
Confocal Microscopy Kristin Jensen 17.11.05 References Cell Biological Applications of Confocal Microscopy, Brian Matsumoto, chapter 1 Studying protein dynamics in living cells,, Jennifer Lippincott-Schwartz
Point Spread Function. Confocal Laser Scanning Microscopy. Confocal Aperture. Optical aberrations. Alternative Scanning Microscopy
 Bi177 Lecture 5 Adding the Third Dimension Wide-field Imaging Point Spread Function Deconvolution Confocal Laser Scanning Microscopy Confocal Aperture Optical aberrations Alternative Scanning Microscopy
Bi177 Lecture 5 Adding the Third Dimension Wide-field Imaging Point Spread Function Deconvolution Confocal Laser Scanning Microscopy Confocal Aperture Optical aberrations Alternative Scanning Microscopy
Training Guide for Carl Zeiss LSM 7 MP Multiphoton Microscope
 Training Guide for Carl Zeiss LSM 7 MP Multiphoton Microscope ZEN 2009 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Turn Chameleon TiS laser key from Standby
Training Guide for Carl Zeiss LSM 7 MP Multiphoton Microscope ZEN 2009 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Turn Chameleon TiS laser key from Standby
Cell Biology and Bioimaging Core
 Cell Biology and Bioimaging Core Leica TCS SP5 Operating Instructions Starting up the instrument 1. First, log in the log book located on the confocal desk. Include your name, your lab s PI, an account
Cell Biology and Bioimaging Core Leica TCS SP5 Operating Instructions Starting up the instrument 1. First, log in the log book located on the confocal desk. Include your name, your lab s PI, an account
MULTIPHOTON MICROSCOPY. Matyas Molnar Dirk Pacholsky
 MULTIPHOTON MICROSCOPY Matyas Molnar Dirk Pacholsky Information Information given here about 2 Photon microscopy were mainly taken from these sources: Background information on 2-Photon microscopy: http://micro.magnet.fsu.edu/primer/techniques/fluorescence/multiphoton/
MULTIPHOTON MICROSCOPY Matyas Molnar Dirk Pacholsky Information Information given here about 2 Photon microscopy were mainly taken from these sources: Background information on 2-Photon microscopy: http://micro.magnet.fsu.edu/primer/techniques/fluorescence/multiphoton/
Leica TCS SP8 Quick Start Guide
 Leica TCS SP8 Quick Start Guide Leica TCS SP8 System Overview Start-Up Procedure 1. Turn on the CTR Control Box, EL6000 fluorescent light source for the microscope stand. 2. Turn on the Scanner Power
Leica TCS SP8 Quick Start Guide Leica TCS SP8 System Overview Start-Up Procedure 1. Turn on the CTR Control Box, EL6000 fluorescent light source for the microscope stand. 2. Turn on the Scanner Power
Training Guide for Carl Zeiss LSM 880 with AiryScan FAST
 Training Guide for Carl Zeiss LSM 880 with AiryScan FAST ZEN 2.3 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2018) Power ON Routine 1 2 Turn ON Main Switch from the remote control
Training Guide for Carl Zeiss LSM 880 with AiryScan FAST ZEN 2.3 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2018) Power ON Routine 1 2 Turn ON Main Switch from the remote control
Basics of confocal imaging (part I)
 Basics of confocal imaging (part I) Swiss Institute of Technology (EPFL) Faculty of Life Sciences Head of BIOIMAGING AND OPTICS BIOP arne.seitz@epfl.ch Lateral resolution BioImaging &Optics Platform Light
Basics of confocal imaging (part I) Swiss Institute of Technology (EPFL) Faculty of Life Sciences Head of BIOIMAGING AND OPTICS BIOP arne.seitz@epfl.ch Lateral resolution BioImaging &Optics Platform Light
Boulevard du Temple Daguerrotype (Paris,1838) a busy street? Nyquist sampling for movement
 Boulevard du Temple Daguerrotype (Paris,1838) a busy street? Nyquist sampling for movement CONFOCAL MICROSCOPY BioVis Uppsala, 2017 Jeremy Adler Matyas Molnar Dirk Pacholsky Widefield & Confocal Microscopy
Boulevard du Temple Daguerrotype (Paris,1838) a busy street? Nyquist sampling for movement CONFOCAL MICROSCOPY BioVis Uppsala, 2017 Jeremy Adler Matyas Molnar Dirk Pacholsky Widefield & Confocal Microscopy
Supplemental Method Information Zeiss LSM710
 Supplemental Method Information Zeiss LSM710 1 Under the Light Path window set up the confocal for imaging a green dye (Alexa488-EGFP). For example, set up the light path as shown here using the 488 nm
Supplemental Method Information Zeiss LSM710 1 Under the Light Path window set up the confocal for imaging a green dye (Alexa488-EGFP). For example, set up the light path as shown here using the 488 nm
Leica TCS SL Confocal Training. Neuroscience Imaging Core Staff. Core Director. Facility Manager
 Leica TCS SL Confocal Training Neuroscience Imaging Facility The Ohio State University Rightmire Hall 614-292-1367 Staff Core Director Anthony Brown, Ph. D. 060 Rightmire Hall 614-292-1205 brown.2302@osu.edu
Leica TCS SL Confocal Training Neuroscience Imaging Facility The Ohio State University Rightmire Hall 614-292-1367 Staff Core Director Anthony Brown, Ph. D. 060 Rightmire Hall 614-292-1205 brown.2302@osu.edu
Leica Sp5 II Confocal User Guide
 Leica Sp5 II Confocal User Guide Turning on the Confocal System (instructions are posted in the room) 1. Turn on Laser Power Button 2. Turn Key to On position 3. Turn on Scanner Power Button 4. Turn on
Leica Sp5 II Confocal User Guide Turning on the Confocal System (instructions are posted in the room) 1. Turn on Laser Power Button 2. Turn Key to On position 3. Turn on Scanner Power Button 4. Turn on
Microscopy from Carl Zeiss
 Microscopy from Carl Zeiss Contents Page Contents... 1 Introduction... 1 Starting the System... 2 Introduction to ZEN Efficient Navigation... 5 Setting up the microscope... 10 Configuring the beam path
Microscopy from Carl Zeiss Contents Page Contents... 1 Introduction... 1 Starting the System... 2 Introduction to ZEN Efficient Navigation... 5 Setting up the microscope... 10 Configuring the beam path
LSM 780 Confocal Microscope Standard Operation Protocol
 LSM 780 Confocal Microscope Standard Operation Protocol Basic Operation Turning on the system 1. Sign on log sheet according to Actual start time 2. Check Compressed Air supply for the air table 3. Switch
LSM 780 Confocal Microscope Standard Operation Protocol Basic Operation Turning on the system 1. Sign on log sheet according to Actual start time 2. Check Compressed Air supply for the air table 3. Switch
Non-Descanned FLIM Detection in Multiphoton Microscopes
 Non-Descanned FLIM Detection in Multiphoton Microscopes Abstract. Multiphoton microscopes use a femtosecond NIR laser to excite fluorescence in the sample. Excitation is performed via a multi-photon absorption
Non-Descanned FLIM Detection in Multiphoton Microscopes Abstract. Multiphoton microscopes use a femtosecond NIR laser to excite fluorescence in the sample. Excitation is performed via a multi-photon absorption
Leica TCS SP8 Quick Start Guide
 Leica TCS SP8 Quick Start Guide Leica TCS SP8 System Overview Start-Up Procedure 1. Turn on the CTR Control Box, Fluorescent Light for the microscope stand. 2. Turn on the Scanner Power (1) on the front
Leica TCS SP8 Quick Start Guide Leica TCS SP8 System Overview Start-Up Procedure 1. Turn on the CTR Control Box, Fluorescent Light for the microscope stand. 2. Turn on the Scanner Power (1) on the front
Resolution. Diffraction from apertures limits resolution. Rayleigh criterion θ Rayleigh = 1.22 λ/d 1 peak at 2 nd minimum. θ f D
 Microscopy Outline 1. Resolution and Simple Optical Microscope 2. Contrast enhancement: Dark field, Fluorescence (Chelsea & Peter), Phase Contrast, DIC 3. Newer Methods: Scanning Tunneling microscopy (STM),
Microscopy Outline 1. Resolution and Simple Optical Microscope 2. Contrast enhancement: Dark field, Fluorescence (Chelsea & Peter), Phase Contrast, DIC 3. Newer Methods: Scanning Tunneling microscopy (STM),
Zeiss LSM 780 Protocol
 Zeiss LSM 780 Protocol 1) System Startup F Please note the sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for unused time.
Zeiss LSM 780 Protocol 1) System Startup F Please note the sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for unused time.
Zeiss LSM 880 Protocol
 Zeiss LSM 880 Protocol 1) System Startup Please note put sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for unused time.
Zeiss LSM 880 Protocol 1) System Startup Please note put sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for unused time.
Shreyash Tandon M.S. III Year
 Shreyash Tandon M.S. III Year 20091015 Confocal microscopy is a powerful tool for generating high-resolution images and 3-D reconstructions of a specimen by using point illumination and a spatial pinhole
Shreyash Tandon M.S. III Year 20091015 Confocal microscopy is a powerful tool for generating high-resolution images and 3-D reconstructions of a specimen by using point illumination and a spatial pinhole
Operating Checklist for using the Laser Scanning Confocal Microscope. Leica TCS SP5.
 Smith College August 2010 Operating Checklist for using the Laser Scanning Confocal Microscope Leica TCS SP5. CONTENT, page no. Startup, 1 Initial set-up, 1 Software, 2 Microscope Specimen observation
Smith College August 2010 Operating Checklist for using the Laser Scanning Confocal Microscope Leica TCS SP5. CONTENT, page no. Startup, 1 Initial set-up, 1 Software, 2 Microscope Specimen observation
CONFOCAL MICROSCOPE (Zeiss LSM 510 META v4.2)
 Wellcome Trust Centre for Human Genetics Molecular Cytogenetics and Microscopy Core CONFOCAL MICROSCOPE (Zeiss LSM 510 META v4.2) 1) STARTING THE SYSTEM Abridged INSTRUCTIONS Switch on the mercury bulb
Wellcome Trust Centre for Human Genetics Molecular Cytogenetics and Microscopy Core CONFOCAL MICROSCOPE (Zeiss LSM 510 META v4.2) 1) STARTING THE SYSTEM Abridged INSTRUCTIONS Switch on the mercury bulb
Examination, TEN1, in courses SK2500/SK2501, Physics of Biomedical Microscopy,
 KTH Applied Physics Examination, TEN1, in courses SK2500/SK2501, Physics of Biomedical Microscopy, 2009-06-05, 8-13, FB51 Allowed aids: Compendium Imaging Physics (handed out) Compendium Light Microscopy
KTH Applied Physics Examination, TEN1, in courses SK2500/SK2501, Physics of Biomedical Microscopy, 2009-06-05, 8-13, FB51 Allowed aids: Compendium Imaging Physics (handed out) Compendium Light Microscopy
BASICS OF CONFOCAL IMAGING (PART I)
 BASICS OF CONFOCAL IMAGING (PART I) INTERNAL COURSE 2012 LIGHT MICROSCOPY Lateral resolution Transmission Fluorescence d min 1.22 NA obj NA cond 0 0 rairy 0.61 NAobj Ernst Abbe Lord Rayleigh Depth of field
BASICS OF CONFOCAL IMAGING (PART I) INTERNAL COURSE 2012 LIGHT MICROSCOPY Lateral resolution Transmission Fluorescence d min 1.22 NA obj NA cond 0 0 rairy 0.61 NAobj Ernst Abbe Lord Rayleigh Depth of field
The Zeiss AiryScan System, Confocal Four.
 The Zeiss AiryScan System, Confocal Four. Overview. The Zeiss AiryScan module is a segmented, radially stacked GaASP detector and collector system designed to subsample the airy disk of a point emission
The Zeiss AiryScan System, Confocal Four. Overview. The Zeiss AiryScan module is a segmented, radially stacked GaASP detector and collector system designed to subsample the airy disk of a point emission
RENISHAW INVIA RAMAN SPECTROMETER
 STANDARD OPERATING PROCEDURE: RENISHAW INVIA RAMAN SPECTROMETER Purpose of this Instrument: The Renishaw invia Raman Spectrometer is an instrument used to analyze the Raman scattered light from samples
STANDARD OPERATING PROCEDURE: RENISHAW INVIA RAMAN SPECTROMETER Purpose of this Instrument: The Renishaw invia Raman Spectrometer is an instrument used to analyze the Raman scattered light from samples
TRAINING MANUAL. Olympus FV1000
 TRAINING MANUAL Olympus FV1000 September 2014 TABLE OF CONTENTS A. Start-Up Procedure... 1 B. Visual Observation under the Microscope... 1 C. Image Acquisition... 4 A brief Overview of the Settings...
TRAINING MANUAL Olympus FV1000 September 2014 TABLE OF CONTENTS A. Start-Up Procedure... 1 B. Visual Observation under the Microscope... 1 C. Image Acquisition... 4 A brief Overview of the Settings...
Invitation for a walk through microscopy. Sebastian Schuchmann Jörg Rösner
 Invitation for a walk through microscopy Sebastian Schuchmann Jörg Rösner joerg.roesner@charite.de Techniques in microscopy Conventional (light) microscopy bright & dark field, phase & interference contrast
Invitation for a walk through microscopy Sebastian Schuchmann Jörg Rösner joerg.roesner@charite.de Techniques in microscopy Conventional (light) microscopy bright & dark field, phase & interference contrast
Contents. Introduction
 Contents Page Contents... 1 Introduction... 1 Starting the System... 2 Introduction to ZEN Efficient Navigation... 5 Setting up the microscope... 10 Configuring the beam path and lasers... 12 Scanning
Contents Page Contents... 1 Introduction... 1 Starting the System... 2 Introduction to ZEN Efficient Navigation... 5 Setting up the microscope... 10 Configuring the beam path and lasers... 12 Scanning
3D light microscopy techniques
 3D light microscopy techniques The image of a point is a 3D feature In-focus image Out-of-focus image The image of a point is not a point Point Spread Function (PSF) 1D imaging 2D imaging 3D imaging Resolution
3D light microscopy techniques The image of a point is a 3D feature In-focus image Out-of-focus image The image of a point is not a point Point Spread Function (PSF) 1D imaging 2D imaging 3D imaging Resolution
b. Turn the power switch and key to on position for blue laser.
 OLYMPUS FLUOVIEW 300 CONFOCAL MICOSCOPE OPERATION PROCEDURE 1. Turn ON microscope in this order: 1) Turn on mercury lamp (Note: once the mercury lamp is turned off, DO NOT turn it back on for at least
OLYMPUS FLUOVIEW 300 CONFOCAL MICOSCOPE OPERATION PROCEDURE 1. Turn ON microscope in this order: 1) Turn on mercury lamp (Note: once the mercury lamp is turned off, DO NOT turn it back on for at least
Leica SP8 TCS Users Manual
 Leica SP8 TCS Users Manual Follow the procedure for start up and log on as posted in the lab. Please log on with your account only and do not share your password with anyone. We track and confirm usage
Leica SP8 TCS Users Manual Follow the procedure for start up and log on as posted in the lab. Please log on with your account only and do not share your password with anyone. We track and confirm usage
MIF ZEISS LSM510 CONFOCAL USER PROTOCOL
 MIF ZEISS LSM510 CONFOCAL USER PROTOCOL START-UP Turn on the Mercury Bulb Power Supply (if needed). Power-on the Control Box. Turn on the computer. Open the LSM 510 software. Choose Scan New Images and
MIF ZEISS LSM510 CONFOCAL USER PROTOCOL START-UP Turn on the Mercury Bulb Power Supply (if needed). Power-on the Control Box. Turn on the computer. Open the LSM 510 software. Choose Scan New Images and
Introduction to light microscopy
 Center for Microscopy and Image Anaylsis Introduction to light microscopy Basic concepts of imaging with light Urs Ziegler ziegler@zmb.uzh.ch Light interacting with matter Absorbtion Refraction Diffraction
Center for Microscopy and Image Anaylsis Introduction to light microscopy Basic concepts of imaging with light Urs Ziegler ziegler@zmb.uzh.ch Light interacting with matter Absorbtion Refraction Diffraction
You won t be able to measure the incident power precisely. The readout of the power would be lower than the real incident power.
 1. a) Given the transfer function of a detector (below), label and describe these terms: i. dynamic range ii. linear dynamic range iii. sensitivity iv. responsivity b) Imagine you are using an optical
1. a) Given the transfer function of a detector (below), label and describe these terms: i. dynamic range ii. linear dynamic range iii. sensitivity iv. responsivity b) Imagine you are using an optical
CMI STANDARD OPERATING PROCEDURE. Fluoview 300 laser scanning confocal microscope
 CMI STANDARD OPERATING PROCEDURE Fluoview 300 laser scanning confocal microscope CMI documentid:sop001 CONTACT INFORMATION: Peter Owens: 091 494036 (office) Peter.owens@nuigalway.ie Kerry Thompson: 091
CMI STANDARD OPERATING PROCEDURE Fluoview 300 laser scanning confocal microscope CMI documentid:sop001 CONTACT INFORMATION: Peter Owens: 091 494036 (office) Peter.owens@nuigalway.ie Kerry Thompson: 091
LSM 800 Confocal Microscope Standard Operation Protocol
 LSM 800 Confocal Microscope Standard Operation Protocol Turning on the system 1. Switch on the Main switch (labeled 1 and 2 ) mounted on the wall. 2. Turn the Laser Key (labeled 3 ) 90 clockwise for power
LSM 800 Confocal Microscope Standard Operation Protocol Turning on the system 1. Switch on the Main switch (labeled 1 and 2 ) mounted on the wall. 2. Turn the Laser Key (labeled 3 ) 90 clockwise for power
Bio 407. Applied microscopy. Introduction into light microscopy. José María Mateos. Center for Microscopy and Image Analysis
 Center for Microscopy and Image Analysis Bio 407 Applied Introduction into light José María Mateos Fundamentals of light Compound microscope Microscope composed of an objective and an additional lens (eyepiece,
Center for Microscopy and Image Analysis Bio 407 Applied Introduction into light José María Mateos Fundamentals of light Compound microscope Microscope composed of an objective and an additional lens (eyepiece,
1 Co Localization and Working flow with the lsm700
 1 Co Localization and Working flow with the lsm700 Samples -1 slide = mousse intestine, Dapi / Ki 67 with Cy3/ BrDU with alexa 488. -1 slide = mousse intestine, Dapi / Ki 67 with Cy3/ no BrDU (but with
1 Co Localization and Working flow with the lsm700 Samples -1 slide = mousse intestine, Dapi / Ki 67 with Cy3/ BrDU with alexa 488. -1 slide = mousse intestine, Dapi / Ki 67 with Cy3/ no BrDU (but with
Microscope Confocal Sp2 Upright.
 Microscope Confocal Sp2 Upright. Welcome to the Leica Sp2 Confocal Upright tutorial. Before using the Sp2 Invert, You will need to put down your name on the reservation system = http://svintranet.epfl.ch/index.php?optio
Microscope Confocal Sp2 Upright. Welcome to the Leica Sp2 Confocal Upright tutorial. Before using the Sp2 Invert, You will need to put down your name on the reservation system = http://svintranet.epfl.ch/index.php?optio
Zeiss LSM880 Operating Instructions. UTMB Optical Microscopy Core Jan. 16, 2018
 Zeiss LSM880 Operating Instructions UTMB Optical Microscopy Core Jan. 16, 2018 1 1. Power up the microscope Sing the LOGBOOK Steps below will provide power to the computer and all of the microscope components.
Zeiss LSM880 Operating Instructions UTMB Optical Microscopy Core Jan. 16, 2018 1 1. Power up the microscope Sing the LOGBOOK Steps below will provide power to the computer and all of the microscope components.
Introduction to light microscopy
 Center for Microscopy and Image Anaylsis Introduction to light Basic concepts of imaging with light Urs Ziegler ziegler@zmb.uzh.ch Microscopy with light 1 Light interacting with matter Absorbtion Refraction
Center for Microscopy and Image Anaylsis Introduction to light Basic concepts of imaging with light Urs Ziegler ziegler@zmb.uzh.ch Microscopy with light 1 Light interacting with matter Absorbtion Refraction
Leica SPEII confocal microscope. Short Manual
 Leica SPEII confocal microscope Short Manual Switching ON sequence: 1. Turn on the Workstation under the bench (top, far right). 2. Turn on the Supply Unit - Laser box (big green switch first and then
Leica SPEII confocal microscope Short Manual Switching ON sequence: 1. Turn on the Workstation under the bench (top, far right). 2. Turn on the Supply Unit - Laser box (big green switch first and then
ZEISS LSM 710 CONFOCAL MICROSCOPE USER MANUAL
 ZEISS LSM 710 CONFOCAL MICROSCOPE USER MANUAL START THE SYSTEM... 2 START ZEN SOFTWARE... 3 SET THE TEMPERATURE AND THE CO2 CONTROLLERS... OBSERVATION AT OCULARS... 5 STATIF PRESENTATION... 6 ACQUIRE ONE
ZEISS LSM 710 CONFOCAL MICROSCOPE USER MANUAL START THE SYSTEM... 2 START ZEN SOFTWARE... 3 SET THE TEMPERATURE AND THE CO2 CONTROLLERS... OBSERVATION AT OCULARS... 5 STATIF PRESENTATION... 6 ACQUIRE ONE
Light Microscopy. Upon completion of this lecture, the student should be able to:
 Light Light microscopy is based on the interaction of light and tissue components and can be used to study tissue features. Upon completion of this lecture, the student should be able to: 1- Explain the
Light Light microscopy is based on the interaction of light and tissue components and can be used to study tissue features. Upon completion of this lecture, the student should be able to: 1- Explain the
LEICA TCS SP5 AOBS TANDEM USER MANUAL
 LEICA TCS SP5 AOBS TANDEM USER MANUAL STARTING THE SYSTEM...2 THE LAS AF SOFTWARE...3 THE «ACQUIRE» MENU...5 CHOOSE AND CREATE A SETTING...6 THE CONTROL PANEL...8 THE DMI6000B MICROSCOPE...10 ACQUIRE ONE
LEICA TCS SP5 AOBS TANDEM USER MANUAL STARTING THE SYSTEM...2 THE LAS AF SOFTWARE...3 THE «ACQUIRE» MENU...5 CHOOSE AND CREATE A SETTING...6 THE CONTROL PANEL...8 THE DMI6000B MICROSCOPE...10 ACQUIRE ONE
Microscopy. Matti Hotokka Department of Physical Chemistry Åbo Akademi University
 Microscopy Matti Hotokka Department of Physical Chemistry Åbo Akademi University What s coming Anatomy of a microscope Modes of illumination Practicalities Special applications Basic microscope Ocular
Microscopy Matti Hotokka Department of Physical Chemistry Åbo Akademi University What s coming Anatomy of a microscope Modes of illumination Practicalities Special applications Basic microscope Ocular
Leica SP8 Resonant Confocal. Quick-Start Guide
 Leica SP8 Resonant Confocal Quick-Start Guide Contents Start-up Preparing for Imaging Part 1 On the scope Part 2 Software interface Part 3 Heat & CO2 incubation Part 4 Other hardware options Shut-down
Leica SP8 Resonant Confocal Quick-Start Guide Contents Start-up Preparing for Imaging Part 1 On the scope Part 2 Software interface Part 3 Heat & CO2 incubation Part 4 Other hardware options Shut-down
5/4/2015 INTRODUCTION TO LIGHT MICROSCOPY. Urs Ziegler MICROSCOPY WITH LIGHT. Image formation in a nutshell. Overview of techniques
 INTRODUCTION TO LIGHT MICROSCOPY Urs Ziegler ziegler@zmb.uzh.ch MICROSCOPY WITH LIGHT INTRODUCTION TO LIGHT MICROSCOPY Image formation in a nutshell Overview of techniques Widefield microscopy Resolution
INTRODUCTION TO LIGHT MICROSCOPY Urs Ziegler ziegler@zmb.uzh.ch MICROSCOPY WITH LIGHT INTRODUCTION TO LIGHT MICROSCOPY Image formation in a nutshell Overview of techniques Widefield microscopy Resolution
ZEISS LSM 710 NLO Multiphoton microscope Manual/Quick guide
 ZEISS LSM 710 NLO Multiphoton microscope Manual/Quick guide Matyas Molnar, Biovis 2016 Starting the microscpe 1. Check the microscope if everything looks clean and normal. If not, report it in the logbook.
ZEISS LSM 710 NLO Multiphoton microscope Manual/Quick guide Matyas Molnar, Biovis 2016 Starting the microscpe 1. Check the microscope if everything looks clean and normal. If not, report it in the logbook.
VISUAL PHYSICS ONLINE DEPTH STUDY: ELECTRON MICROSCOPES
 VISUAL PHYSICS ONLINE DEPTH STUDY: ELECTRON MICROSCOPES Shortly after the experimental confirmation of the wave properties of the electron, it was suggested that the electron could be used to examine objects
VISUAL PHYSICS ONLINE DEPTH STUDY: ELECTRON MICROSCOPES Shortly after the experimental confirmation of the wave properties of the electron, it was suggested that the electron could be used to examine objects
Guide to Confocal 5. Starting session
 Guide to Confocal 5 Remember that when booking and before starting session you can check for any problems at https://www.bris.ac.uk/biochemistry/uobonly/cif/index.html Starting session Switch on microscope
Guide to Confocal 5 Remember that when booking and before starting session you can check for any problems at https://www.bris.ac.uk/biochemistry/uobonly/cif/index.html Starting session Switch on microscope
Nikon. King s College London. Imaging Centre. N-SIM guide NIKON IMAGING KING S COLLEGE LONDON
 N-SIM guide NIKON IMAGING CENTRE @ KING S COLLEGE LONDON Starting-up / Shut-down The NSIM hardware is calibrated after system warm-up occurs. It is recommended that you turn-on the system for at least
N-SIM guide NIKON IMAGING CENTRE @ KING S COLLEGE LONDON Starting-up / Shut-down The NSIM hardware is calibrated after system warm-up occurs. It is recommended that you turn-on the system for at least
INTRODUCTION TO MICROSCOPY. Urs Ziegler THE PROBLEM
 INTRODUCTION TO MICROSCOPY Urs Ziegler ziegler@zmb.uzh.ch THE PROBLEM 1 ORGANISMS ARE LARGE LIGHT AND ELECTRONS: ELECTROMAGNETIC WAVES v = Wavelength ( ) Speed (v) Frequency ( ) Amplitude (A) Propagation
INTRODUCTION TO MICROSCOPY Urs Ziegler ziegler@zmb.uzh.ch THE PROBLEM 1 ORGANISMS ARE LARGE LIGHT AND ELECTRONS: ELECTROMAGNETIC WAVES v = Wavelength ( ) Speed (v) Frequency ( ) Amplitude (A) Propagation
Supplemental Figure 1: Histogram of 63x Objective Lens z axis Calculated Resolutions. Results from the MetroloJ z axis fits for 5 beads from each
 Supplemental Figure 1: Histogram of 63x Objective Lens z axis Calculated Resolutions. Results from the MetroloJ z axis fits for 5 beads from each lens with a 1 Airy unit pinhole setting. Many water lenses
Supplemental Figure 1: Histogram of 63x Objective Lens z axis Calculated Resolutions. Results from the MetroloJ z axis fits for 5 beads from each lens with a 1 Airy unit pinhole setting. Many water lenses
Confocal imaging on the Leica TCS SP8. 1) Turn the system on. 2) Use TCS user account. 3) Start LAS X software:
 Confocal imaging on the Leica TCS SP8 1) Turn the system on. 2) Use TCS user account. 3) Start LAS X software: 4) Do not touch the microscope while the software is initializing. Choose your options: Turn
Confocal imaging on the Leica TCS SP8 1) Turn the system on. 2) Use TCS user account. 3) Start LAS X software: 4) Do not touch the microscope while the software is initializing. Choose your options: Turn
Confocal Laser Scanning Microscopy
 Name of the Core Facility: Confocal Laser Scanning Microscopy CORE Forschungszentrum Immunologie Mainz Welcome to the CSLM Core Facility: The CLSM Core Facility enables working groups to incorporate high
Name of the Core Facility: Confocal Laser Scanning Microscopy CORE Forschungszentrum Immunologie Mainz Welcome to the CSLM Core Facility: The CLSM Core Facility enables working groups to incorporate high
Confocal 510 Tutorial
 Confocal 510 Tutorial You will have to log on in order to use the microscope. You will be charged according the time you are logged in, so please don t forget to log out after you are done. If you don
Confocal 510 Tutorial You will have to log on in order to use the microscope. You will be charged according the time you are logged in, so please don t forget to log out after you are done. If you don
Introduction to light microscopy
 Center for Microscopy and Image Anaylsis Introduction to light Imaging with light / Overview of techniques Urs Ziegler ziegler@zmb.uzh.ch Light interacting with matter Absorbtion Refraction Diffraction
Center for Microscopy and Image Anaylsis Introduction to light Imaging with light / Overview of techniques Urs Ziegler ziegler@zmb.uzh.ch Light interacting with matter Absorbtion Refraction Diffraction
Microscopic Structures
 Microscopic Structures Image Analysis Metal, 3D Image (Red-Green) The microscopic methods range from dark field / bright field microscopy through polarisation- and inverse microscopy to techniques like
Microscopic Structures Image Analysis Metal, 3D Image (Red-Green) The microscopic methods range from dark field / bright field microscopy through polarisation- and inverse microscopy to techniques like
Title: Leica SP5 Confocal User Manual
 Title: Leica SP5 Confocal User Manual Date of first issue: 23/10/2015 Date of review: Version: Admin For assistance or to report an issue Office: CG07 or 05 Email: Igmm-imaginghelpdesk@igmm.ed.ac.uk Website:
Title: Leica SP5 Confocal User Manual Date of first issue: 23/10/2015 Date of review: Version: Admin For assistance or to report an issue Office: CG07 or 05 Email: Igmm-imaginghelpdesk@igmm.ed.ac.uk Website:
Fundamentals of Light Microscopy II: Fluorescence, Deconvolution, Confocal, Multiphoton, Spectral microscopy. Integrated Microscopy Course
 Fundamentals of Light Microscopy II: Fluorescence, Deconvolution, Confocal, Multiphoton, Spectral microscopy Integrated Microscopy Course Review Lecture 1: Microscopy Basics Light train Kohler illumination*
Fundamentals of Light Microscopy II: Fluorescence, Deconvolution, Confocal, Multiphoton, Spectral microscopy Integrated Microscopy Course Review Lecture 1: Microscopy Basics Light train Kohler illumination*
Imaging Retreat - UMASS Customized real-time confocal and 2-photon imaging
 Imaging Retreat - UMASS 2012 Customized real-time confocal and 2-photon imaging Mike Sanderson Department of Microbiology and Physiological Systems University of Massachusetts Medical School Thanks for
Imaging Retreat - UMASS 2012 Customized real-time confocal and 2-photon imaging Mike Sanderson Department of Microbiology and Physiological Systems University of Massachusetts Medical School Thanks for
Multifluorescence The Crosstalk Problem and Its Solution
 Multifluorescence The Crosstalk Problem and Its Solution If a specimen is labeled with more than one fluorochrome, each image channel should only show the emission signal of one of them. If, in a specimen
Multifluorescence The Crosstalk Problem and Its Solution If a specimen is labeled with more than one fluorochrome, each image channel should only show the emission signal of one of them. If, in a specimen
a) How big will that physical image of the cells be your camera sensor?
 1. Consider a regular wide-field microscope set up with a 60x, NA = 1.4 objective and a monochromatic digital camera with 8 um pixels, properly positioned in the primary image plane. This microscope is
1. Consider a regular wide-field microscope set up with a 60x, NA = 1.4 objective and a monochromatic digital camera with 8 um pixels, properly positioned in the primary image plane. This microscope is
MIF ZEISS VIOLET CONFOCAL ZEN 2009 PROTOCOL
 MIF ZEISS VIOLET CONFOCAL ZEN 2009 PROTOCOL START-UP On the Switchbox, turn both black switches to the ON position. Wait for the microscope to boot up completely (watch the screen on the side of the microscope).
MIF ZEISS VIOLET CONFOCAL ZEN 2009 PROTOCOL START-UP On the Switchbox, turn both black switches to the ON position. Wait for the microscope to boot up completely (watch the screen on the side of the microscope).
Introduction to light microscopy
 Center for Microscopy and Image Anaylsis Introduction to light microscopy (an overview) Microscopy with light Components of a light microscope 1. Light source 2. Objective 3. Sample or specimen holder
Center for Microscopy and Image Anaylsis Introduction to light microscopy (an overview) Microscopy with light Components of a light microscope 1. Light source 2. Objective 3. Sample or specimen holder
How to align your laser for two-photon imaging
 How to align your laser for two-photon imaging Two-photon microscopy uses a laser to excite fluorescent molecules (fluorophores) within a sample through emitting short pulses of light at high power. This
How to align your laser for two-photon imaging Two-photon microscopy uses a laser to excite fluorescent molecules (fluorophores) within a sample through emitting short pulses of light at high power. This
長庚大學共軛焦顯微鏡課程 長庚大學共軛焦顯微鏡課程. Spot light 長庚大學
 長庚大學共軛焦顯微鏡課程 Spot light 長庚大學共軛焦顯微鏡課程 20071030 長庚大學 Basic principle of Laser Scanning Confocal Microscopy The application of LSM 510 META detector Multiphoton microscopy basic principle and introduction
長庚大學共軛焦顯微鏡課程 Spot light 長庚大學共軛焦顯微鏡課程 20071030 長庚大學 Basic principle of Laser Scanning Confocal Microscopy The application of LSM 510 META detector Multiphoton microscopy basic principle and introduction
Olympus Confocal Microscope User Guide Last updated
 1 Olympus Confocal Microscope User Guide Last updated 11-22-2017 Contents: Laser Safety Training Requirement... 1 Mercury Lamp Precautions... 1 Biosafety requirements and rules for work in the MIC... 2
1 Olympus Confocal Microscope User Guide Last updated 11-22-2017 Contents: Laser Safety Training Requirement... 1 Mercury Lamp Precautions... 1 Biosafety requirements and rules for work in the MIC... 2
