Leginon: An automated system for acquisition of images from vitreous ice specimens.
|
|
|
- Lisa Hutchinson
- 5 years ago
- Views:
Transcription
1 Leginon: An automated system for acquisition of images from vitreous ice specimens. Bridget Carragher, Nick Kisseberth,? David Kriegman, *Ronald A. Milligan, Clinton S. Potter, James Pulokas, Amy Reilein Beckman Institute, and? Department of Computer Science, University of Illinois at Urbana-Champaign, Urbana, Illinois and *The Scripps Research Institute, La Jolla, California Corresponding Author: Bridget Carragher Beckman Institute for Advanced Science and Technology 405 N. Mathews Avenue Urbana, Illinois, Tel: ; Fax: ; Abstract We have developed a system to automatically acquire cryo-electron micrographs. The system is designed to emulate all of the decisions and actions of a highly trained microscopist in collecting data from a vitreous ice specimen. These include identifying suitable areas of vitreous ice at low magnification, determining the presence and location of specimen on the grid, automatically adjusting imaging parameters (focus, astigmatism) under low dose conditions and acquiring images at high magnification to either film or a digital camera. This system is responsible for every aspect of image acquisition and can run unattended, other than requiring periodic refilling of the cryogens, for over 24 hours. The system has been tested out on a variety of specimens which represent typical challenges in the field of cryo-electron microscopy. The results show that the overall performance of the system is equivalent to that of an experienced microscopist. Keywords: TEM, cryo-electron microscopy, automation.
2 Introduction Cryo-electron microscopy is proving to be one of the most important structural approaches in cell biological investigations. The method relies on the acquisition and analysis of large numbers of electron micrographs of macromolecular structures, which are usually preserved in vitreous ice. However, despite the obvious utility of these techniques they have not been routinely applied in large numbers of laboratories. Part of the explanation lies in the difficulty and tedium associated with collecting sufficient data to complete a three dimensional reconstruction. In general the techniques associated with cryo-electron microscopy are challenging to master and require frequent practice to keep up a reasonable level of expertise. Furthermore, in order to determine structures to high resolution, very large numbers of images must be acquired. The exact number needed depends on the size of the macromolecule and the desired resolution of the reconstruction. These numbers have been estimated (Henderson, 1995) and while some of the details of these estimates remain under discussion, it is generally agreed that in order to interpret a structure to atomic resolution data from hundreds of thousands of copies of the macromolecule must contribute to the average. Depending on the number of macromolecules imaged on each micrograph, this in turn requires the collection of thousands to tens of thousands of electron micrographs. The current state of the art requires that these images be acquired manually by a highly trained microscopist. We propose that automated methods for data collection and analysis will have a significant impact in transferring the cryo-electron microscopy technology to the general 2
3 biological community as well as in increasing the volume of data that can be collected during a single session at the microscope. We have thus developed an automated system that can emulate all of the decisions and actions of a highly trained microscopist in collecting data from a vitreous ice specimen. This includes identifying suitable areas of vitreous ice at low magnification, determining the presence and location of specimen on the grid, automatically adjusting imaging parameters (focus, astigmatism) under low dose conditions and acquiring images at high magnification to either film or a digital camera. This system can run unattended for over 24 hours and is responsible for every aspect of image acquisition; the only intervention required of the operator is the periodic refilling of cryogens. While there are a number of other semi-automated systems (Oostergetel et al., 1998; Stewart et al., 2000) that relieve the operator of some of the tedious tasks associated with data acquisition, in these systems the operator needs to identify suitable areas of the grid, select the location for each image to be acquired, and provide input to the system during the data acquisition process. Thus, while these systems may be useful in practice, they do not solve the problem that an expert microscopist is required in attendance at the microscope at all times. Our goal in this project has been to develop a system that can be easily set up and left unattended to continuously acquire images. In this paper, we will describe the underlying methods used to develop the system and to evaluate its performance on several test specimens. The results show that the data collected by the automated system is comparable in quality to data collected by a human operator. 3
4 Methods Specimen Preparation In cryo-electron microscopy (Dubochet et al., 1988), images are obtained of specimens preserved in vitreous ice and suspended across a hole in the supporting carbon substrate. Grids are prepared by blotting a buffer containing the specimen to a thin film and plunging the film rapidly into liquid ethane. Optimal imaging conditions are based on the size of the hole and thickness and quality of the ice. We have used holey grids provided by Quantifoil Inc. (Ermantraut et al., 1998) as our specimen substrate. These grids provide holes of fixed size and geometry and greatly simplify the algorithms required for automated identification of the holes. Specifically we used holes of 2um diameter spaced either 4um or 6um apart. Routine preparation of grids that are covered by ice of optimal thickness ( A) remains a challenge even for experienced microscopists. Frequently, there are only limited areas of the grid that contain ice of suitable quality and thickness. Other problems are encountered in evenly distributing the specimen across the grid. If the density of the specimen is low, the yield of images that contain specimen is correspondingly low. On the other hand, if the density of the specimen is too high it is not possible to isolate individual macromolecules in the images for further analysis. One of the tasks of a microscopist, and thus of an automated system, is to examine the grid at very low magnification in order to identify areas that are suitable for further examination and imaging. 4
5 Microscopy The automated system has been developed on a Philips CM200 transmission electron microscope interfaced to a Gatan 1Kx1Kx12bit MSC CCD camera. Currently the system runs in two separate facilities; the Beckman Institute for Advanced Science and Technology in Illinois and the Scripps Research Institute in La Jolla where the CM200 is equipped with a field emission gun. Both microscopes are equipped with a Gatan cryotransfer system. The CM200 is controlled by a UNIX workstation running the emscope software library (Kisseberth et al., 1997) and connected via a serial port to the microscope. The Gatan camera is controlled through a plug in to the Digital Micrograph program running on a Macintosh workstation. Computation The overall software system, called Leginon, runs on a UNIX workstation. A variety of different versions of Unix have been used during the course of these experiments (IBM AIX, SGI Irix, Linux) and the code runs transparently on any of them. The user interface to the system has been developed using the Tcl/Tk scripting language (Ousterhout, 1994) interfaced to the viewit image processing package (Potter and Moran, 1992). The interface communicates to the TEM using Tcl extensions to the emscope software library (Kisseberth et al., 1997) and to the Gatan camera over a network connection to the Digital Micrograph plug in. Our design goals were to keep the system modular and flexible and to reuse as much existing code as possible in order to minimize development time. Tcl/Tk was used to develop the user interface as it is well supported on Unix platforms and includes an optional graphical user interface. The system can also be 5
6 monitored using a web browser interface that displays the current status of the experiment and the last set of acquired images. This is useful in that a long experiment can be monitored from any convenient workstation, which might be located in nearby office or from any remote location. Automated collection of cryo-electron micrographs Overview Our goal was to develop a system that would reproduce the actions of a skilled microscopist in acquiring images from vitreous ice specimens. As translated into a series of steps performed by our automated system, these tasks are outlined in figure 1 and illustrated by images in figure 2. Briefly, vitreous ice specimens are prepared over Quantifoil grids (Ermantraut et al., 1998) that provide a holey substrate with a welldefined geometry. The specimen grid is inserted into the microscope and a short sequence of calibrations and alignments are performed by the operator to define the geometry of the grid and the preferred collection parameters for the specimen. The automated system then systematically scans the grid starting with the collection of a low magnification image of each grid square (figure 2a). The low magnification image is first examined to determine if the grid square is intact and free of contamination. The grid square is then analyzed to identify target holes that contain ice of suitable thickness. For sparsely distributed specimens, an image of each target hole is then acquired at an intermediate magnification (figure 2b). This image is analyzed to detect the presence of specimen and, optionally to target the location of the best specimen within the area. 6
7 Procedures for focusing and adjusting astigmatism, as well as ensuring that the specimen is not drifting, are next performed under low dose conditions. Finally, a high magnification image is acquired, either to film or to the digital camera (figure 2c). In each of the sections below we will describe the technique used and provide some results as to the performance of the system in practice. Initial Calibration and Setup During initial calibration and setup, the instrument settings which define parameters for searching the grid, focusing, and acquiring a high magnification image under low dose conditions are set and recorded. We have developed a set of tools that assist the user in defining the low dose imaging conditions. These tools provide semi-automated methods for calibrating image shifts that are used to maintain the image of the specimen at the center of the viewing screen as the magnification changes. Maintaining a feature of interest and the electron beam in the center of the field of view as the magnification changes repeatedly over several orders of magnitude posed unexpected difficulties. Hysteresis effects in the electromagnets on the electron microscope column resulted in shifts in the image and beam over time during protracted data collection runs. If the images were being collected manually this would not pose a problem as the operator would simply tweak the settings occasionally to recenter the image and the beam but we wanted to remove this necessity. The major cause of hysteresis resulted from changing to very low magnifications (660x) because the objective lens of the microscope is turned off at this magnification. We solved this problem by working the engineers at Philips to reprogram the lens currents on the microscope column so that we could reach the low 7
8 magnification settings without turning off the objective lens. In addition we also always fully saturate the lenses after every magnification change. The relative beam and image positions are now stable over a 24 hour period during which the magnification is changed thousands of times. As part of the grid calibration step, a number of parameters are measured and recorded. These include the orientation of the grid bars; the geometrical arrangement of the holes; and the selection of a desired range of ice thickness to be used when selecting target areas where high magnification images will be recorded. At the beginning of each session stored values which define the calibrations for automatically setting focus and astigmatism can also be checked. However, we have found that these settings are extremely stable and do not usually need to be recalibrated. We have standardized most of the setup procedures so that each session starts out with the previous sessions parameters as a starting point. Currently the initial setup and calibration steps take less than a half hour to complete. Low Magnification Image Acquisition and Analysis After the initial calibration is completed the next step is to identify individual grid squares that are intact, uncontaminated and contain holes with suitable vitreous ice. In the automated system this is achieved by acquiring and analyzing a low magnification (660x) image of an entire grid square (figure 2a). There are between 500 to 1000 individual squares on the grids that we typically use. Images of each square can either be acquired systematically starting from a central point or the operator may define certain 8
9 areas of the grid where data acquisition is preferred. Each low magnification image is analyzed to assess the overall integrity of the grid square (is the substrate torn or damaged? is the ice contaminated?) and to identify target hole locations for subsequent image acquisition at higher magnifications. Suitable target holes will contain specimens embedded in uncontaminated ice of the correct thickness. Within each grid square, holes are identified by correlation template matching followed by a procedure to iteratively determine the geometrical parameters that describe the Quantifoil lattice and estimate the thickness of the ice (Carragher et al., 1999). The algorithm to identify holes includes steps to (i) edge sharpen the image using a Laplacian mask; (ii) correlate the image with a synthetic template of an edge sharpened hole; (iii) determine the coordinates of each hole center by finding a local maximum in the correlation map; (iv) check if the hole coordinates fit the local lattice that defines the hole geometry; and (v) calculate the mean and variance of the image intensity within each hole to provide an estimate of ice thickness and consistency. The size of the template is based on the diameter of the hole and is set during the initial calibration procedures. One of the advantages of the automated system is that it provides a quantitative measure of the ice thickness that is reproducible from session to session. The thickness of a vitreous ice layer can be estimated as (Eusemann et al., 1982; Lepault et al., 1982): t? K ln(i 0 /I) where I 0 is the intensity of a bright field image in the absence of ice and I is the intensity of the image in the presence of an ice layer of thickness t. K is a constant that is 9
10 dependent on the geometry of the microscope. For our microscope operating at 120KeV we estimate K to be approximately 1?m. The unattenuated beam intensity is measured during the initial calibration step. We use this relationship to set parameters on the automated hole finder to find only those areas of the grid which contain ice of a specified thickness. The automated hole identification technique described is extremely robust even for grids where the carbon foil has been damaged and the geometrical lattice distorted. The method appears to be very reproducible between sessions and between specimens and requires minimal modification of parameters by the user. Visual inspection confirms that, of the hole locations identified, over 95% are appropriate targets. The parameters which are used to select ice thickness have been optimized to mimic the range of ice thickness that would be chosen by an experienced microscopist. We estimate that the parameters that we currently use represent a range of ice thickness from nm. We have found that once these parameters are established they can consistently be used between experiments to select ice of a predictable thickness. Intermediate Magnification Analysis and Targeting Once holes containing ice of suitable thickness have been identified, the hole must be located at the center of the field of view, and a decision must be made as to whether the hole is suitable for further analysis. The accuracy with which the selected hole can be relocated to the center of the field of view depends on the accuracy with which the goniometer on the TEM can be moved to an absolute location. The accuracy of the 10
11 Philips compustage (Asselbergs et al., 1993) is only on the order of 1um and this is about an order of magnitude less than required for the automated system. To achieve the required accuracy an approach was developed in which the slew rate of the goniometer is precisely characterized and used to model its behavior (Pulokas et al., 2000). Using this model, any hole within a single grid square can be positioned to the center of the field of view with an accuracy of about 0.1um. For specimens that are uniformly and densely distributed across the grid every hole is likely to contain appropriate specimen and the center of the hole thus provides a suitable target location for high magnification image acquisition. However, there are several circumstances where it is necessary to identify the presence of specimen within a hole and, optionally, to accurately locate individual targets for imaging within the hole. One of the applications we have been pursuing is automated acquisition of images of microtubules. A difficulty in acquiring sufficient numbers of these images is that they are often sparsely distributed across a grid and only a small percentage of the holes might contain any specimen. For this reason, each suitable hole must be analyzed in order to determine the presence or absence of filaments. Without this analysis a large percentage of the acquired images would not contain a useful specimen. If the high magnification image is acquired to a large field of view detector, like film, the entire area of the hole can be captured and thus it is only necessary to detect the presence of specimen, not target individual filaments. However, if the high magnification images are acquired with a limited field of view detector, like a CCD camera, then the specimen must be accurately 11
12 located at the center of the field of view or once again the acquired image will most likely not contain a useful specimen. We have developed an automated technique to detect and target specimens within each hole using the intermediate magnification image (figure 2b). The method calculates correlation maps between this image and a bank of 36 filament templates. The templates in the bank are constructed from a short segment of an individual filament model, which is then rotated by five degrees over 0 to 180 degrees. The magnitude and distribution of the correlations between the image and the bank of templates provides information about the presence as well as the orientation of individual filaments. For each pixel, a weighted function of the correlations is computed and compared to a threshold. A variation of the Hough transform (Trucco and Verri, 1998) is finally used to locate long straight sections of filament. A detailed summary of the filament detection algorithm is as follows: (i) Filter the image with a Laplacian mask that responds strongly to intensity discontinuities (edges). (ii) Mask the areas of the image outside of the hole. (iii) Correlate the image with the bank of synthetic templates containing short segments of a filtered filament in 36 orientations. (iv) For each pixel location, compute the mean and standard deviation of the 36 filter responses. (v) Create a weighted maximum correlation map by finding the maximum response of the 36 filters and z-scoring this value (subtract the mean and divide by the standard deviation). This also provides a rough estimate of the filament's orientation. (vi) Threshold the weighted maximum correlation map. (vii) Identify straight line segments in the map from step (vi) using a variation of the Hough transform which takes account of input pixel coordinates as well as local orientation. (viii) Select 12
13 the targeting locations at the center of the longest straight line segment. Use the length of the detected filament as a criterion for determining if a suitable filament is present. The result of the intermediate magnification analysis is a set of coordinates within the hole where there are likely to be filaments of reasonable length and small curvature. If the final high magnification images are to be acquired to film, these results are used simply to decide whether to expose the sheet of film. If the final images are to be acquired with a CCD camera, the coordinates of the center of the straightest identified filament are used to locate that filament to the center of the field of view at high magnification. Visual inspection of the intermediate magnification images has confirmed that the filament finding algorithm accurately identifies holes containing specimen with better than a 90% accuracy. When acquiring images of microtubules to film, this ensures that there is a high yield of micrographs that contain filaments. The filament finding algorithm has also been used to target long straight segments along the identified filaments for acquisition to the CCD camera. The targeting algorithm accurately locates the filament to within 20 nm of the center of the field of view. This ensures that the specimen in contained within the area of the CCD during the high magnification digital imaging acquisition. 13
14 Focus and Astigmatism Correction Once a decision to acquire a high magnification image has been made the specimen must be examined to ensure that it is not drifting and the focus and astigmatism must be adjusted. Drift measurement, focusing and astigmatism correction procedures are all performed by the automated system under low dose conditions (i.e. using an area of the specimen adjacent to the area that will ultimately be imaged and analyzed). The area we use is located between the holes where the background substrate provides some structural detail that aids in these measurements. To determine drift, images are acquired at short intervals and cross correlation techniques are used to measure the displacement between these images. These drift measurements will continue until the measured displacement is less than a user specified value (e.g. 0.2nm/s). If the drift rate fails to fall below a desired value, the system moves onto the next hole and the operator is notified. The automated focus and astigmatism correction procedures use well established methods based on beam tilt induced image shifts (Dierksen et al., 1993; Dierksen et al., 1992; Fung et al., 1996; Koster et al., 1992; Koster and Ruijter, 1992). This method relies on the fact that the amount of image displacement resulting from an induced beam tilt is linearly related to the amount of defocus. Two images are recorded using beam tilts separated by several mrads and the displacement between them is measured using cross correlation techniques. The automated focusing is implemented in two steps. The initial step measures the focus and based on this measurement sets the defocus to a user 14
15 specified value (e.g. 1um). A second measurement in then made and the final desired defocus value is set. If the results of these measurements are anomalous, the automated system will move to an alternative low dose focus position and try again. If this second position also produces anomalous results, the system moves onto the next hole and notifies the operator. To evaluate the performance of the automated focus correction algorithm we directly measured the defocus set by the algorithm using power spectra of images acquired at the low dose focus position (i.e. offset from the high magnification target). The structure of the contrast transfer function (CTF) is clearly visible in these spectra and by measuring the location of the zeroes in the CTF the defocus of the image can be calculated (Erickson and Klug, 1971; Toyoshima and Unwin, 1988). We used the results of an experiment conducted using the CM200-FEG at the Scripps Institute to acquire this data because the high coherence of the field emission gun makes the zeroes in the CTF very easy to locate. The results show that we can accurately set defocus to within +/-50 nm (n=112) on a vitreous ice specimen even when the flatness of the grid requires shifts of many microns between target positions. This performance is comparable to that of an experienced microscopist. High Magnification Acquisition High magnification images can be acquired either to film or a digital CCD camera. When shooting to film our current practice is to acquire the entire target area within the hole onto a single sheet of film at a magnification of about 38,000x under low dose 15
16 conditions (<10e - /A 2 ). The researcher then develops, prints and examines the image to identify areas that are suitable for subsequent digitization and further analysis. The resolution of the film is such that it can be digitized to provide information to an effective resolution of about 5A. When using the CCD camera for high magnification acquisition, the identified target specimen is centered in the field of view using the image shift coils on the microscope. We estimate that the effective resolution in the acquired digital image at 38,000x is approximately 15A and at 66,000x is approximately 10A (this is approximately 2/3 the Nyquist sampling frequency of the CCD). These estimates of resolution are based on acquired images of catalase crystals and helical TMV filaments. Overall operation of the system User interface The series of steps described above is setup and controlled using a graphical user interface written in Tcl/Tk and running on a Unix platform. The main interface to the system, illustrated in figure 3a, provides overall control of the experiment and information on the current status. It allows the user to specify how images should be saved and analyzed and whether the results of any procedures should be manually confirmed. It is also used to launch a number of subsidiary stand alone tools, including an interface to set up the low dose kit, and manage the selection of grid squares. 16
17 The low dose setup interface allows for the creation of various preset imaging conditions similar to the low dose mode buttons (search, focus, exposure) on the TEM console. An unlimited number of presets may be recorded and saved. For each of these preset conditions we also record specifications for the CCD camera configuration used to record the image. These include exposure time, size, and binning factor. In addition, if the camera sensitivity has been calibrated, the electron dose at each preset imaging condition is measured and recorded. The selection of grid squares to be systematically examined is managed using the grid window, illustrated in figure 3b. The user is required to perform a simple calibration that records the orientation and size of the grid squares and the interface then displays a map of the grid squares that are accessible within the range of the goniometer. The current imaging position is indicated on this map and the operator can navigate around the grid using the mouse. The interface is also used to specify a protocol for automatically scanning the grid. The default option is to systematically spiral out from the current position but the operator can also specify particular paths or individual grid squares. Areas that the operator wishes to avoid and those that have already been imaged by the Leginon system are crossed off. All options and parameters displayed on the control windows can be modified interactively while data acquisition is in progress. If the process is halted, or the system crashes unexpectedly, the interface can be restarted and the system will be restored, allowing the process to start up exactly where it left off. The system can also be 17
18 monitored using a web browser interface that displays the current status of the experiment and the last set of acquired images. When the system needs attention or problems are encountered, this information is automatically sent to the operator s account and optionally to an alphanumeric pager. There are two cryogenic systems that need regular replenishment of liquid nitrogen (LN2): the anticontamination device on the column of the CM-200 and the Gatan cryostage that maintains the temperature of the vitreous ice specimen. Several years ago, we extended the life of the LN2 dewar which cools the anti-contamination device. This was achieved very simply by changing the size of the dewar (from 1 liter to 2 liter) and designing a new attachment to the microscope to support the larger container. The new container lasts up to 12 hours before it needs to be refilled. Extending the life of the cryostage poses far greater difficulties. The Gatan cryoholder has a capacity of less than 0.25 liter of LN2, and the volume of liquid in the holder is critical to maintain a stable temperature and minimizing drift in the specimen. Any automated refilling system must maintain the level of LN2 while not adding any vibration to the system or any excessive weight to the cryostage. Our initial approach has been to develop a simple refilling system that allows the specimen to be kept cold but does not require that perfect imaging conditions be maintained. This allows the operator to pause the experiment overnight while maintaining the temperature of the grid (Robinson et al., 2000). 18
19 Dosimetry The final high magnification images can be acquired either to film or the digital camera. The total dose accumulated by the specimen is always kept to approximately 10e/A 2, typical of the accepted norm for low dose imaging conditions. An example of typical accumulated doses at the specimen for various stages of image acquisition are as follows: Magnification Dose (e/a 2 ) , The dose received by the image at low magnification is negligible. At intermediate magnification the dose is less than 0.3 e/a2. However, due to the spread of the beam and the standard configuration of the Quantifoil grid (2um diameter holes on 4um spacing), holes adjacent to the hole that is currently being imaged might receive up to four exposures of the intermediate magnification dose (for a total of approximately 1e/A 2 ). We have since redesigned the geometry of our Quantifoil grids (2 um holes spaced 6 um apart) to avoid the problem of multiple exposures. The remaining total accumulated dose may be budgeted for one or more high magnification image acquisitions. Timing Some typical running times for the Leginon system operating on a 700 MhZ Pentium III PC under Linux v 2.2. are as follows: 19
20 Step Low magnification acquisition Timing 10 s Hole identification 5 Intermediate magnification acquisition 10 Specimen targeting 15 Drift check 40 Focus and astigmatism correction 15 High magnification acquisition 30 These timings are not optimized for fast data collection and include the acquisition, analysis and storage of several intermediate images that we currently use to assess the performance of the system. The rate at which high magnification images can be acquired is not only dependent on the speed of acquisition and analysis but more importantly on the quality of the specimen grid. Both the integrity of the Quantifoil grid, the quality of the ice, and the density and distribution of the specimen all affect the rate at which data can be acquired. For example, the system may spend time acquiring and analyzing intermediate magnification images of holes that have ice of the right thickness but have no specimen in them. Applications We have tested and evaluated the performance of the Leginon system by applying it to three specific applications that represent typical challenges faced by cryo-electron microscopists. 20
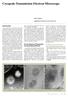
21 Single Particles: Cow Pea Mosaic Virus Spherical viruses represent a class of specimens that have proved very tractable to analysis using cryo electron microscopy. Several groups have now achieved reconstructions of viruses to resolutions of better than 10A. This resolution could be further improved by increasing the total number of images contributing to the three dimensional map. We used the Leginon system to acquire images of cow pea mosaic virus, an icosahedral virus with a capsid size of approximately 300A (Lin et al., 1999). Data was continuously collected over a period of 19 hours during which time a total of 50 grid squares were examined, 458 holes containing ice of suitable thickness were identified on these grid squares, and 439 high magnification (38,000x) images were acquired to the CCD camera. As the specimen was well dispersed across the grid and viruses were present in every hole, no intermediate magnification acquisition or analysis was performed to determine the presence of specimen or to target its location within a given hole. Instead, high magnification images were acquired at the center of each identified hole. An example of one of these images is shown in figure 4. The images were visually inspected and evaluated and we estimated that 350 of them contained data that was suitable for further analysis. As each image contained approximately viruses, the experiment potentially provided about 12,000 particles that could be used for a three dimensional reconstruction. 21
22 Helical Filaments: Tobacco Mosaic Virus. Tobacco mosaic virus (TMV) is a well characterized helical virus that has a repeating structure such that there are strong layer lines in the power spectrum at 11.5A and 23A. It is often used as a TEM standard for calibration of magnification and determining resolution and provides an ideal test specimen for evaluating a new technique. The Leginon system was used to acquire images of these helical filaments in several experimental sessions at the microscope. In one of these, the high magnification images were acquired at a final magnification of 66000x. Over the 19 hours of the experiment, a total of 212 grid squares were examined and 359 holes were identified as having ice of suitable thickness. Filaments were identified and targeted in 249 of these holes and a total of 215 high magnification images were digitally acquired at a magnification of 66,000x and a nominal defocus of 400nm. An example of an image and its power spectrum are shown in figures 6a and b. The 11.5 A layer line is clearly visible indicating that the CCD, at least at 120KeV and for moderate resolutions, provides a viable alternative to film provided that the individual filaments can be accurately targeted. Helical Filaments: Microtubules In order to reconstruct an electron density map of microtubules at 20-30A a sufficient number of images must be averaged together. Previous work shows that ~30 images of helical microtubules are needed to calculate a reliable 3D map at ~20A resolution (Sosa et al., 1997). Acquiring these images can be a challenging task. Microtubules are linear polymers with a wide range of lengths and achieving a good even distribution of them on 22
23 a frozen grid is difficult; variable amounts are removed from the grid during the blotting stage of grid preparation. In addition, only about 5% of the microtubules are helical and thus to get the ~30 images necessary for calculating a structure, ~600 microtubule images must be collected. Prints are then made from these images and the helical microtubules identified by visual examination. The selected images are then scanned on a microdensitiometer to provide optical density arrays for digital image analysis. Clearly this procedure is labor intensive and time consuming. The ability to automatically identify microtubules suitable for analysis during image acquisition and to collect these images in digital format would increase productivity dramatically. Here we describe two experiments to demonstrate the feasibility of automating the acquisition of these images. The first experiment used the Philips CM-200 FEG at the Scripps Research Institute. Leginon was used to systematically scan areas of the grid that were identified by the operator as potentially containing suitable specimens. Seven grid squares were evaluated and 80 holes containing suitable ice were identified on these grid squares. An evaluation of the hole at intermediate magnification (6600x) was performed in order to detect the presence of filaments within the hole and 59 were identified as containing filaments. A total of 49 high magnification images (38,000x) of these holes were acquired to film and a second image was then subsequently acquired to the digital camera. The images acquired to film were processed and the films were visually inspected and analyzed on an optical bench. The optical diffraction pattern of a large area of the film was examined to identify any overall problems with drift, focus or astigmatism. The optical diffraction patterns of individual masked filaments were also inspected to determine if the 40A layer 23
24 line of the microtubule was visible. This is the procedure normally used for post analysis when acquiring films using the standard manual acquisition procedures. Of the 49 films acquired, a total of 38 were acceptable in terms of imaging conditions and contained filaments that diffracted well. The remaining 11 films had various problems associated with drift or astigmatism. A second experiment to acquire images of microtubules used the CM200 located at the Beckman Institute, UIUC, and used the digital camera for all high magnification image acquisition. Data was collected continuously over a period of 17 hours; a total of 68 grid squares were examined and 544 holes were identified as containing suitable ice. The intermediate magnification targeting algorithm was used to identify the presence and location of filaments within the hole. The longest, straightest filament within each hole was targeted for high magnification digital acquisition. A total of 235 filaments were targeted in this way and 226 high magnification images were subsequently acquired using the CCD camera. The 9 images of targeted filaments which were not acquired were due to problems associated with drift or the inability of the automated algorithm to set a suitable defocus value. Several examples of images acquired this way are in figure 7. Discussion Our goal in this paper has been to demonstrate that we have developed an automated system that can perform all of the tasks normally performed by a trained microscopist in acquiring images from vitreous ice specimens. The Leginon system is routinely used on 24
25 a weekly basis at the Beckman Institute and has been used at the Scripps Institute to collect data on a number of occasions. The amount of time required to set up the system and begin acquiring data is less than one hour and this time includes aligning the microscope and setting up the low dose kit as well as initial calibrations for grid and hole geometry and ice thickness. We are now at the stage that once the system has been setup and begins to acquire data, no intervention is required on the part of the operator other than to refill the cryogens. Typically, we continuously acquire data for 12 to 18 hours but the system has been run on several occasions for over 30 hours. We tested the Leginon system on a variety of specimens. The experiment using cow pea mosaic virus demonstrated that the system could acquire 12,000 images of single particles in a single session. We are confident that we could increase the number of acquired images by at least a factor of 4. The application experiments with TMV demonstrated that the system could acquire large numbers of images of helical filaments to the CCD with a resolution of approximately 10A at a magnification of 66,000x. For the microtubules, images were acquired both to film and the CCD. Visual inspection of the films judged that the overall performance of the system was equivalent to that of a highly experienced microscopist. Digital images acquired using the CCD demonstrated the potential of the system for replacing film and thus bypassing many of the tedious and time consuming steps associated with acquiring sufficient data for a three dimensional map. In either case the automated system has some advantages in providing instantaneous feedback as to the quality of the specimen, drift rate, defocus settings etc. 25
26 It also allows an experiment to be monitored from any location, for example, the experiment running in Illinois is frequently monitored from Scripps in California. We have made the results of one of the automated experiments available for viewing over the web. In this experiment, images of TMV were acquired at 66,000x. These images along with associated low and intermediate magnification images can be viewed at Cryoelectron microscopy has traditionally been a difficult technique that few laboratories have mastered. Acquiring high quality images of specimens embedded in vitreous ice normally requires substantial training and regular practice. Our goal was to provide a system that would allow a relatively inexperienced operator to routinely acquire good quality micrographs of specimens embedded in vitreous ice. The Leginon system is currently being used in two laboratories. At the Beckman Institute, where the system was developed, it is normally set up and managed by an individual with no training in microscopy. In contrast, at the Scripps Research Institute, the system is managed by a microscopist with no special training with computers. Both individuals can effectively use the system to obtain good quality micrographs. The Legion system can automatically acquire images to film and thus emulate the current system of acquiring data. However, since the operator must manually change the film cassette every 56 plates this is not ideal for a high throughput operation. Clearly, CCD cameras are a preferable medium for high magnification image acquisition. The principal disadvantage is the limited area of the specimen imaged by the digital detector as 26
27 compared to film at equivalent resolution. From our preliminary results we believe that a 2Kx2K camera combined with the specimen targeting algorithm will allow us to collect data directly to the CCD and effectively eliminate the need for film. At this stage of development of the Leginon system the time required to automatically acquire a micrograph is longer that required by an experienced microscopist to perform the same task. This is particularly true when the specimen is sparsely dispersed, as a microscopist is faster at locating the specimens and the good imaging areas on the grid. We are planning to improve the performance of the system by improving computational efficiency and moving the computationally intensive algorithms to a parallel computer architecture. We are also developing more intelligent algorithms to speed up identification of suitable areas of the grid. Our goal is to improve the acquisition time by at least a factor of 4. With improved timings and a larger area CCD we are confident that we will be able to automatically acquire images directly to the CCD of high quality and with an efficiency that will make the automated collection techniques a viable and attractive alternative to manual data acquisition. 27
28 Acknowledgements The authors wish to thank Dr. Jack Johnson at Scripps Research Institute for providing the cow pea mosaic virus. This project was funded by the National Science Foundation under grants DBI and DBI
29 Figures Figure 1: Schematic overview of the Leginon system. Figure 2: (a) Low magnification (660x) image acquisition. Holes that are identified as containing ice of suitable thickness are indicated by crosses. (b) Intermediate magnification (6600x) image of the hole indicated by an arrow in (a). (c) High magnification (66000x) image taken from the area indicated by a cross in (b). Figure 3: Graphical user interface of the Leginon system. (a) Main user interface provides overall control of the experiment and information on the current status. (b) The grid scan interface. The current imaging position is indicated by a circle. The operator can navigate around the grid using the mouse and can specify a path to be used in systematically scanning the grid. Areas that the operator wishes to avoid and those that have already been imaged by Leginon are crossed off. Figure 4: Image of cow pea mosaic virus acquired using the CCD camera at 38,000x. Figure 5: (a) An image of TMV acquired using the CCD camera at a magnification of 38000x. (b) A power spectrum of the image in (a) indicates the presence of the 23A layer line. Figure 6: (a) Image of TMV acquired using the CCD camera at a magnification of 66000x. (b) Power spectrum of the filament indicated by the arrow in (a). The small arrow indicates the 23A layer line and the large arrow the 11.5A layer line. Figure 7: Images of microtubules acquired using the CCD camera at a magnification of 38,000x 29
30 References Asselbergs, S.J., J. Brock, M.J.C.d. Jong, and M.T. Otten The New CM-Series TEMs: Integration of a Five-Axis Motorized, Fully Computer-Controlled Goniometer. Inst. Phys. Conf.: Carragher, B., N. Jojic, R.A. Milligan, N. Kisseberth, J. Pulokas, C.S. Potter, and A. Reilein Automated Acquisition of Cryo-Electron Micrographs. Microscopy and Microanalysis 99. 5: Dierksen, K., D. Typke, R. Hegerl, and W. Baumeister Towards Automatic Electron Tomography II: Implementation of Autofocus and Low-Dose Procedures. Ultramicroscopy. 49: Dierksen, K., D. Typke, R. Hegrl, A.J. Koster, and W. Baumeister Towards Automatic Electron Tomography. Ultramicroscopy. 40: Dubochet, J., M. Adrian, J.J. Chang, J.C. Homo, J. Lepault, A.W. McDowall, and P. Schultz Cryoelectron Microscopy of Vitrified Specimens. Quart. Rev. Biophys. 21: Erickson, H.P., and A. Klug Measurement and compensation of defocusing and aberrations by Fourier processing of electron micrographs. Phil. Trans. Roy. Soc. Lond. B. 261: Ermantraut, E., K. Wohlfart, and W. Tichelaar Perforated support foils with redefined hole size, shape and arrangement. Ultramicroscopy. 74: Eusemann, R., H. Rose, and J. Dubochet Electron Scattering in Ice and Organic Materials. Journal of Microscopy. 128:
31 Fung, J.C., W. Liu, W.J. DeRuijter, H. Chen, C.K. Abbey, J.W. Sedat, and D.A. Agard Toward Fully Automated High-Resolution Electron Tomography. Journal of Structural Biology. 116: Henderson, R The Potential and Limitations of Neutrons, Electrons, and X-rays for Atomic Resolution Microscopy of Unstained Biological Macromolecules. Quarterly Review of Biophysics. 28: Kisseberth, N., M. Whittaker, D. Weber, C.S. Potter, and B. Carragher emscope: A Tool Kit for Control and Automation of a Remote Electron Microscope. Journal of Structural Biology. 120: Koster, A.J., H. Chen, J.W. Sedat, and D.A. Agard Automated Microscopy for Electron Tomography. Ultramicroscopy. 46: Koster, A.J., and W.J.d. Ruijter Practical Autoalignment of Transmission Electron Microscopes. Ultramicroscopy. 40: Lepault, J., F.P. Booy, and J. Dubochet Electron Microscopy of Frozen Biological Suspensions. Journal of Microscopy. 129: Lin, T., Z. Chen, R. Usha, C.V. Stauffacher, J.-B. Dai, T. Schmidt, and J.E. Johnson The refined crystal structure of cowpea mosaic virus at 2.8 angtroms resolution. Virology. 265: Oostergetel, G.T., W. Keegstra, and A. Brisson Automation of specimen selection and data acquisition for protein crystallography. Ultramicroscopy. 40: Ousterhout, J.K Tcl and the Tk Toolkit. Addison-Wesley. Potter, C.S., and P.J. Moran Viewit: A Software System for Multi-Dimensional Biomedical Image Processing, Analysis, and Visualization. In SPIE Conference 31
32 on Biomedical Image Processing III and Three-Dimensional Microscopy. Vol Pulokas, J., C. Green, N. Kisseberth, C.S. Potter, and B. Carragher Improving the Positional Accuracy of the Goniometer on the Philips CM Series TEM. Journal of Structural Biology. 128: Robinson, S.J., G. Fried, and J. Pulokas An automated system for maintaining liquid nitrogen levels in the Gatan cryostage. In Proceedings of Microscopy and Microanalysis 2000, Phildelphia, PA Sosa, H., D.P. Dias, A. Hoenger, M. Whittaker, E. Wilson-Kubalek, E. Sablin, R.J. Fletterick, R.D. Vale, and R.A. Milligan A Model for the Microtubule- NCD Motor Protein Complex Obtained by Cryo-Electron Microscopy and Image Analysis. Cell. 90: Stewart, P.L., R.B. Cary, S.R. Peterson, and C.Y. Chiu Digitally Collected Cryo- Electron Micrographs for Single Particle Reconstruction. Microscopy Research and Technique. 49: Toyoshima, C., and N. Unwin Contrast transfer for frozen-hydrated specimens: determination from pairs of defoused images. Ultramicroscopy. 25: Trucco, E., and A. Verri Introductory techniques for 3-D computer vision. Simon & Schuster, Upper Saddle River, New Jersey. 32
33 Calibration and alignment Define grid scan protocol Low magnification image acquisition (660x) Low magnification image analysis -Identify location of holes -Estimate ice thickness Intermediate magnification image acquisition (6600x) Intermediate magnification image analysis -Estimate probability of specimen -Identify location of best specimen Drift check, focus and astigmatism correction High magnification image acquisition (38,000x) Figure 1: Schematic overview of the Leginon system.
34 a. b. c. Figure 2: (a) Low magnification (660x) image acquisition. Holes that are identified as containing ice of suitable thickness are indicated by crosses. (b) Intermediate magnification (6600x) image of the hole indicated by an arrow in (a). (c) High magnification (66000x) image taken from the area indicated by a cross in (b).
35 a. b. Figure 3: Graphical user interface of the Leginon system. (a) Main user interface provides overall control of the experiment and information on the current status. (b) The grid scan interface. The current imaging position is indicated by a circle. The operator can navigate around the grid using the mouse and can specify a path to be used in systematically scanning the grid. Areas that the operator wishes to avoid and those that have already been imaged by Leginon are crossed off.
36 Figure 4: Image of cow pea mosaic virus acquired using the CCD camera at 38,000x.
37 a. b. Figure 5: (a) An image of TMV acquired using the CCD camera at a magnification of 38000x. (b) A power spectrum of the image in (a) indicates the presence of the 23A layer line.
38 a. b. Figure 6: (a) Image of TMV acquired using the CCD camera at a magnification of 66000x. (b) Power spectrum of the filament indicated by the arrow in (a). The small arrow indicates the 23A layer line and the large arrow the 11.5A layer line.
39 Figure 7: Images of microtubules acquired using the CCD camera at a magnification of 38,000x
Procedures for Performing Cryoelectron Microscopy on the FEI Sphera Microscope
 Procedures for Performing Cryoelectron Microscopy on the FEI Sphera Microscope The procedures given below were written specifically for the FEI Tecnai G 2 Sphera microscope. Modifications will need to
Procedures for Performing Cryoelectron Microscopy on the FEI Sphera Microscope The procedures given below were written specifically for the FEI Tecnai G 2 Sphera microscope. Modifications will need to
Contrast transfer. Contrast transfer and CTF correction. Lecture 6 H Saibil
 Lecture 6 H Saibil Contrast transfer Contrast transfer and CTF correction The weak phase approximation Contrast transfer function Determining defocus CTF correction methods Image processing for cryo microscopy
Lecture 6 H Saibil Contrast transfer Contrast transfer and CTF correction The weak phase approximation Contrast transfer function Determining defocus CTF correction methods Image processing for cryo microscopy
Cryo-Electron Microscopy of Viruses
 Blockkurs Biophysic and Structural Biology 2013 Praktikumsversuch at C-CINA Cryo-Electron Microscopy of Viruses In this practical we will compare electron microscopy of negatively stained and frozen-hydrated
Blockkurs Biophysic and Structural Biology 2013 Praktikumsversuch at C-CINA Cryo-Electron Microscopy of Viruses In this practical we will compare electron microscopy of negatively stained and frozen-hydrated
Automated acquisition of cryo-electron micrographs for single particle reconstruction on an FEI Tecnai electron microscope
 Journal of Structural Biology 150 (2005) 69 80 www.elsevier.com/locate/yjsbi Automated acquisition of cryo-electron micrographs for single particle reconstruction on an FEI Tecnai electron microscope Jianlin
Journal of Structural Biology 150 (2005) 69 80 www.elsevier.com/locate/yjsbi Automated acquisition of cryo-electron micrographs for single particle reconstruction on an FEI Tecnai electron microscope Jianlin
Phase plates for cryo-em
 Max Planck Institute of Biochemistry Martinsried, Germany MAX PLANCK SOCIETY Phase plates for cryo-em Rado Danev Max Planck Institute of Biochemistry, Martinsried, Germany. EMBO course 2017, London, UK
Max Planck Institute of Biochemistry Martinsried, Germany MAX PLANCK SOCIETY Phase plates for cryo-em Rado Danev Max Planck Institute of Biochemistry, Martinsried, Germany. EMBO course 2017, London, UK
No part of this material may be reproduced without explicit written permission.
 This material is provided for educational use only. The information in these slides including all data, images and related materials are the property of : Robert M. Glaeser Department of Molecular & Cell
This material is provided for educational use only. The information in these slides including all data, images and related materials are the property of : Robert M. Glaeser Department of Molecular & Cell
Recent results from the JEOL JEM-3000F FEGTEM in Oxford
 Recent results from the JEOL JEM-3000F FEGTEM in Oxford R.E. Dunin-Borkowski a, J. Sloan b, R.R. Meyer c, A.I. Kirkland c,d and J. L. Hutchison a a b c d Department of Materials, Parks Road, Oxford OX1
Recent results from the JEOL JEM-3000F FEGTEM in Oxford R.E. Dunin-Borkowski a, J. Sloan b, R.R. Meyer c, A.I. Kirkland c,d and J. L. Hutchison a a b c d Department of Materials, Parks Road, Oxford OX1
XTEM. --Software for Complex Transmission Electron Microscopy. Version 1.0
 XTEM --Software for Complex Transmission Electron Microscopy Version 1.0 1. Introduction XTEM is the software for complex microscopy on JEOL 3100 electron microscopes. The XTEM software consists of a suite
XTEM --Software for Complex Transmission Electron Microscopy Version 1.0 1. Introduction XTEM is the software for complex microscopy on JEOL 3100 electron microscopes. The XTEM software consists of a suite
FEI Falcon Direct Electron Detector. Best Practice Document
 FEI Falcon Direct Electron Detector Best Practice Document 2 1. Introduction FEI Falcon Direct Electron Detector Best Practice Application Guide The FEI Falcon Detector is based on direct electron detection
FEI Falcon Direct Electron Detector Best Practice Document 2 1. Introduction FEI Falcon Direct Electron Detector Best Practice Application Guide The FEI Falcon Detector is based on direct electron detection
Transmission Electron Microscopy 9. The Instrument. Outline
 Transmission Electron Microscopy 9. The Instrument EMA 6518 Spring 2009 02/25/09 Outline The Illumination System The Objective Lens and Stage Forming Diffraction Patterns and Images Alignment and Stigmation
Transmission Electron Microscopy 9. The Instrument EMA 6518 Spring 2009 02/25/09 Outline The Illumination System The Objective Lens and Stage Forming Diffraction Patterns and Images Alignment and Stigmation
Introduction of New Products
 Field Emission Electron Microscope JEM-3100F For evaluation of materials in the fields of nanoscience and nanomaterials science, TEM is required to provide resolution and analytical capabilities that can
Field Emission Electron Microscope JEM-3100F For evaluation of materials in the fields of nanoscience and nanomaterials science, TEM is required to provide resolution and analytical capabilities that can
STEM Spectrum Imaging Tutorial
 STEM Spectrum Imaging Tutorial Gatan, Inc. 5933 Coronado Lane, Pleasanton, CA 94588 Tel: (925) 463-0200 Fax: (925) 463-0204 April 2001 Contents 1 Introduction 1.1 What is Spectrum Imaging? 2 Hardware 3
STEM Spectrum Imaging Tutorial Gatan, Inc. 5933 Coronado Lane, Pleasanton, CA 94588 Tel: (925) 463-0200 Fax: (925) 463-0204 April 2001 Contents 1 Introduction 1.1 What is Spectrum Imaging? 2 Hardware 3
Image Contrast Theory
 Image Contrast Theory Wah Chiu wah@bcm.tmc.edu National Center for Macromolecular Imaging References Jiang, W. & Chiu, W. Web-based simulation for contrast transfer function and envelope functions. Microsc
Image Contrast Theory Wah Chiu wah@bcm.tmc.edu National Center for Macromolecular Imaging References Jiang, W. & Chiu, W. Web-based simulation for contrast transfer function and envelope functions. Microsc
Low Voltage Electron Microscope
 LVEM5 Low Voltage Electron Microscope Nanoscale from your benchtop LVEM5 Delong America DELONG INSTRUMENTS COMPACT BUT POWERFUL The LVEM5 is designed to excel across a broad range of applications in material
LVEM5 Low Voltage Electron Microscope Nanoscale from your benchtop LVEM5 Delong America DELONG INSTRUMENTS COMPACT BUT POWERFUL The LVEM5 is designed to excel across a broad range of applications in material
Cryogenic Transmission Electron Microscope
 Cryogenic Transmission Electron Microscope Hideo Nishioka Application & Research Center, JEOL Ltd. Introduction The transmission electron microscope (TEM) that has been widely used in research in the fields
Cryogenic Transmission Electron Microscope Hideo Nishioka Application & Research Center, JEOL Ltd. Introduction The transmission electron microscope (TEM) that has been widely used in research in the fields
Nature Methods: doi: /nmeth Supplementary Figure 1. Resolution of lysozyme microcrystals collected by continuous rotation.
 Supplementary Figure 1 Resolution of lysozyme microcrystals collected by continuous rotation. Lysozyme microcrystals were visualized by cryo-em prior to data collection and a representative crystal is
Supplementary Figure 1 Resolution of lysozyme microcrystals collected by continuous rotation. Lysozyme microcrystals were visualized by cryo-em prior to data collection and a representative crystal is
ELECTRON MICROSCOPY. 13:10 16:00, Oct. 6, 2008 Institute of Physics, Academia Sinica. Tung Hsu
 ELECTRON MICROSCOPY 13:10 16:00, Oct. 6, 2008 Institute of Physics, Academia Sinica Tung Hsu Department of Materials Science and Engineering National Tsing Hua University Hsinchu 300, TAIWAN Tel. 03-5742564
ELECTRON MICROSCOPY 13:10 16:00, Oct. 6, 2008 Institute of Physics, Academia Sinica Tung Hsu Department of Materials Science and Engineering National Tsing Hua University Hsinchu 300, TAIWAN Tel. 03-5742564
SCIENTIFIC INSTRUMENT NEWS. Introduction. Design of the FlexSEM 1000
 SCIENTIFIC INSTRUMENT NEWS 2017 Vol. 9 SEPTEMBER Technical magazine of Electron Microscope and Analytical Instruments. Technical Explanation The FlexSEM 1000: A Scanning Electron Microscope Specializing
SCIENTIFIC INSTRUMENT NEWS 2017 Vol. 9 SEPTEMBER Technical magazine of Electron Microscope and Analytical Instruments. Technical Explanation The FlexSEM 1000: A Scanning Electron Microscope Specializing
FEI Tecnai G 2 F20 Operating Procedures
 FEI Tecnai G 2 F20 Operating Procedures 1. Startup (1) Sign-up in the microscope log-sheet. Please ensure you have written an account number for billing. (2) Log in to the computer: Login to your account
FEI Tecnai G 2 F20 Operating Procedures 1. Startup (1) Sign-up in the microscope log-sheet. Please ensure you have written an account number for billing. (2) Log in to the computer: Login to your account
Automated acquisition of electron microscopic random conical tilt sets
 Journal of Structural Biology 157 (2006) 148 155 Journal of Structural Biology www.elsevier.com/locate/yjsbi Automated acquisition of electron microscopic random conical tilt sets Shawn Q. Zheng a,b, Justin
Journal of Structural Biology 157 (2006) 148 155 Journal of Structural Biology www.elsevier.com/locate/yjsbi Automated acquisition of electron microscopic random conical tilt sets Shawn Q. Zheng a,b, Justin
2 How to operate the microscope/obtain an image
 Morgagni Operating Instructions 50079 010912 2-1 2 ow to operate the microscope/obtain an image 2.1 Starting the microscope 2.1.1 Starting the microscope with several manually-operated steps 1. Turn on
Morgagni Operating Instructions 50079 010912 2-1 2 ow to operate the microscope/obtain an image 2.1 Starting the microscope 2.1.1 Starting the microscope with several manually-operated steps 1. Turn on
1.2. Make sure the viewing screen is covered (exposure to liquid N 2 may cause it to crack).
 FEI Tecnai F20 S/TEM: imaging in TEM mode Nicholas G. Rudawski ngr@ufl.edu (805) 252-4916 (352) 392-3077 Last updated: 01/21/18 1. Filling the cold trap (if needed) 1.1. Prior to use, the cold trap needs
FEI Tecnai F20 S/TEM: imaging in TEM mode Nicholas G. Rudawski ngr@ufl.edu (805) 252-4916 (352) 392-3077 Last updated: 01/21/18 1. Filling the cold trap (if needed) 1.1. Prior to use, the cold trap needs
Low Voltage Electron Microscope. Nanoscale from your benchtop LVEM5. Delong America
 LVEM5 Low Voltage Electron Microscope Nanoscale from your benchtop LVEM5 Delong America DELONG INSTRUMENTS COMPACT BUT POWERFUL The LVEM5 is designed to excel across a broad range of applications in material
LVEM5 Low Voltage Electron Microscope Nanoscale from your benchtop LVEM5 Delong America DELONG INSTRUMENTS COMPACT BUT POWERFUL The LVEM5 is designed to excel across a broad range of applications in material
2014 HTD-E with options
 with options The HT7700 : a user-friendly, ergonomic digital TEM with options User-Friendly r end Design Ambient light operation. Multiple automated functions for alignment, focus and stigmation as standard
with options The HT7700 : a user-friendly, ergonomic digital TEM with options User-Friendly r end Design Ambient light operation. Multiple automated functions for alignment, focus and stigmation as standard
Low Voltage Electron Microscope
 LVEM 25 Low Voltage Electron Microscope fast compact powerful Delong America FAST, COMPACT AND POWERFUL The LVEM 25 offers a high-contrast, high-throughput, and compact solution with nanometer resolutions.
LVEM 25 Low Voltage Electron Microscope fast compact powerful Delong America FAST, COMPACT AND POWERFUL The LVEM 25 offers a high-contrast, high-throughput, and compact solution with nanometer resolutions.
Very short introduction to light microscopy and digital imaging
 Very short introduction to light microscopy and digital imaging Hernan G. Garcia August 1, 2005 1 Light Microscopy Basics In this section we will briefly describe the basic principles of operation and
Very short introduction to light microscopy and digital imaging Hernan G. Garcia August 1, 2005 1 Light Microscopy Basics In this section we will briefly describe the basic principles of operation and
General principles of image processing in cryo-em
 Lecture 13 E. Orlova Birkbeck College, London General principles of image processing in cryo-em Cryo EM & 3D Image Processing 8 July 2016 Thiruvananthapuram, India William Lawrence Bragg Crystallography
Lecture 13 E. Orlova Birkbeck College, London General principles of image processing in cryo-em Cryo EM & 3D Image Processing 8 July 2016 Thiruvananthapuram, India William Lawrence Bragg Crystallography
LVEM 25. Low Voltage Electron Mictoscope. fast compact powerful
 LVEM 25 Low Voltage Electron Mictoscope fast compact powerful FAST, COMPACT AND POWERFUL The LVEM 25 offers a high-contrast, high-throughput, and compact solution with nanometer resolutions. All the benefits
LVEM 25 Low Voltage Electron Mictoscope fast compact powerful FAST, COMPACT AND POWERFUL The LVEM 25 offers a high-contrast, high-throughput, and compact solution with nanometer resolutions. All the benefits
Full-screen mode Popup controls. Overview of the microscope user interface, TEM User Interface and TIA on the left and EDS on the right
 Quick Guide to Operating FEI Titan Themis G2 200 (S)TEM: TEM mode Susheng Tan Nanoscale Fabrication and Characterization Facility, University of Pittsburgh Office: M104/B01 Benedum Hall, 412-383-5978,
Quick Guide to Operating FEI Titan Themis G2 200 (S)TEM: TEM mode Susheng Tan Nanoscale Fabrication and Characterization Facility, University of Pittsburgh Office: M104/B01 Benedum Hall, 412-383-5978,
Basic Users Manual for Tecnai-F20 TEM
 Basic Users Manual for Tecnai-F20 TEM NB: This document contains my personal notes on the operating procedure of the Tecnai F20 and may be used as a rough guide for those new to the microscope. It may
Basic Users Manual for Tecnai-F20 TEM NB: This document contains my personal notes on the operating procedure of the Tecnai F20 and may be used as a rough guide for those new to the microscope. It may
Low-energy Electron Diffractive Imaging for Three dimensional Light-element Materials
 Low-energy Electron Diffractive Imaging for Three dimensional Light-element Materials Hitachi Review Vol. 61 (2012), No. 6 269 Osamu Kamimura, Ph. D. Takashi Dobashi OVERVIEW: Hitachi has been developing
Low-energy Electron Diffractive Imaging for Three dimensional Light-element Materials Hitachi Review Vol. 61 (2012), No. 6 269 Osamu Kamimura, Ph. D. Takashi Dobashi OVERVIEW: Hitachi has been developing
A Laser-Based Thin-Film Growth Monitor
 TECHNOLOGY by Charles Taylor, Darryl Barlett, Eric Chason, and Jerry Floro A Laser-Based Thin-Film Growth Monitor The Multi-beam Optical Sensor (MOS) was developed jointly by k-space Associates (Ann Arbor,
TECHNOLOGY by Charles Taylor, Darryl Barlett, Eric Chason, and Jerry Floro A Laser-Based Thin-Film Growth Monitor The Multi-beam Optical Sensor (MOS) was developed jointly by k-space Associates (Ann Arbor,
Development of JEM-2800 High Throughput Electron Microscope
 Development of JEM-2800 High Throughput Electron Microscope Mitsuhide Matsushita, Shuji Kawai, Takeshi Iwama, Katsuhiro Tanaka, Toshiko Kuba and Noriaki Endo EM Business Unit, JEOL Ltd. Electron Optics
Development of JEM-2800 High Throughput Electron Microscope Mitsuhide Matsushita, Shuji Kawai, Takeshi Iwama, Katsuhiro Tanaka, Toshiko Kuba and Noriaki Endo EM Business Unit, JEOL Ltd. Electron Optics
Automated Identification of Filaments in Cryo-electron Microscopy Images
 Automated Identification of Filaments in Cryo-electron Microscopy Images Yuanxin Zhu, Bridget Carragher, David Kriegman, Ronald A. Milligan, and Clinton S. Potter (Submitted to Journal of Structural Biology)
Automated Identification of Filaments in Cryo-electron Microscopy Images Yuanxin Zhu, Bridget Carragher, David Kriegman, Ronald A. Milligan, and Clinton S. Potter (Submitted to Journal of Structural Biology)
ELECTRON MICROSCOPY. 14:10 17:00, Apr. 3, 2007 Department of Physics, National Taiwan University. Tung Hsu
 ELECTRON MICROSCOPY 14:10 17:00, Apr. 3, 2007 Department of Physics, National Taiwan University Tung Hsu Department of Materials Science and Engineering National Tsinghua University Hsinchu 300, TAIWAN
ELECTRON MICROSCOPY 14:10 17:00, Apr. 3, 2007 Department of Physics, National Taiwan University Tung Hsu Department of Materials Science and Engineering National Tsinghua University Hsinchu 300, TAIWAN
The End of Thresholds: Subwavelength Optical Linewidth Measurement Using the Flux-Area Technique
 The End of Thresholds: Subwavelength Optical Linewidth Measurement Using the Flux-Area Technique Peter Fiekowsky Automated Visual Inspection, Los Altos, California ABSTRACT The patented Flux-Area technique
The End of Thresholds: Subwavelength Optical Linewidth Measurement Using the Flux-Area Technique Peter Fiekowsky Automated Visual Inspection, Los Altos, California ABSTRACT The patented Flux-Area technique
Quantitative HRTEM investigation of an obtuse angle dislocation reaction in gold with a C S corrected field emission microscope
 Quantitative HRTEM investigation of an obtuse angle dislocation reaction in gold with a C S corrected field emission microscope Joerg R. Jinschek 1, Ch. Kisielowski 1,2, T. Radetic 1, U. Dahmen 1, M. Lentzen
Quantitative HRTEM investigation of an obtuse angle dislocation reaction in gold with a C S corrected field emission microscope Joerg R. Jinschek 1, Ch. Kisielowski 1,2, T. Radetic 1, U. Dahmen 1, M. Lentzen
--> Buy True-PDF --> Auto-delivered in 0~10 minutes. JY/T
 Translated English of Chinese Standard: JY/T011-1996 www.chinesestandard.net Sales@ChineseStandard.net INDUSTRY STANDARD OF THE JY PEOPLE S REPUBLIC OF CHINA General rules for transmission electron microscopy
Translated English of Chinese Standard: JY/T011-1996 www.chinesestandard.net Sales@ChineseStandard.net INDUSTRY STANDARD OF THE JY PEOPLE S REPUBLIC OF CHINA General rules for transmission electron microscopy
Appreciating the very little things: Status and future prospects of TEM at NUANCE
 Appreciating the very little things: Status and future prospects of TEM at NUANCE Dr. Roberto dos Reis roberto.reis@northwestern.edu 11/28/2018 Nature 542, pages75 79 (2017) TEM Facility Manager: Dr. Xiaobing
Appreciating the very little things: Status and future prospects of TEM at NUANCE Dr. Roberto dos Reis roberto.reis@northwestern.edu 11/28/2018 Nature 542, pages75 79 (2017) TEM Facility Manager: Dr. Xiaobing
LowDose for JEOL. --Software LowDose and Advanced Data Acquisition. Version 1.1
 LowDose for JEOL --Software LowDose and Advanced Data Acquisition Version 1.1 1. Introduction This is the LowDose software for the user of JEOL electron microscopes. The LowDose software is a Plug-In for
LowDose for JEOL --Software LowDose and Advanced Data Acquisition Version 1.1 1. Introduction This is the LowDose software for the user of JEOL electron microscopes. The LowDose software is a Plug-In for
LVEM 25. Low Voltage Electron Microscope Fast Compact Powerful.... your way to electron microscopy
 LVEM 25 Low Voltage Electron Microscope Fast Compact Powerful... your way to electron microscopy INTRODUCING THE LVEM 25 High Contrast & High Resolution Unmatched contrast of biologic and light material
LVEM 25 Low Voltage Electron Microscope Fast Compact Powerful... your way to electron microscopy INTRODUCING THE LVEM 25 High Contrast & High Resolution Unmatched contrast of biologic and light material
Instructions for Tecnai a brief start up manual
 Instructions for Tecnai a brief start up manual Version 3.0, 8.12.2015 Manual of Tecnai 12 transmission electron microscope located at Aalto University's Nanomicroscopy Center. More information of Nanomicroscopy
Instructions for Tecnai a brief start up manual Version 3.0, 8.12.2015 Manual of Tecnai 12 transmission electron microscope located at Aalto University's Nanomicroscopy Center. More information of Nanomicroscopy
Operating the Hitachi 7100 Transmission Electron Microscope Electron Microscopy Core, University of Utah
 Operating the Hitachi 7100 Transmission Electron Microscope Electron Microscopy Core, University of Utah Follow the procedures below when you use the Hitachi 7100 TEM. Starting Session 1. Turn on the cold
Operating the Hitachi 7100 Transmission Electron Microscope Electron Microscopy Core, University of Utah Follow the procedures below when you use the Hitachi 7100 TEM. Starting Session 1. Turn on the cold
ECEN 4606, UNDERGRADUATE OPTICS LAB
 ECEN 4606, UNDERGRADUATE OPTICS LAB Lab 2: Imaging 1 the Telescope Original Version: Prof. McLeod SUMMARY: In this lab you will become familiar with the use of one or more lenses to create images of distant
ECEN 4606, UNDERGRADUATE OPTICS LAB Lab 2: Imaging 1 the Telescope Original Version: Prof. McLeod SUMMARY: In this lab you will become familiar with the use of one or more lenses to create images of distant
contents TABLE OF The SECOM platform Applications - sections Applications - whole cells Features Integrated workflow Automated overlay
 S E C O M TABLE OF contents The SECOM platform 4 Applications - sections 5 Applications - whole cells 8 Features 9 Integrated workflow 12 Automated overlay ODEMIS - integrated software Specifications 13
S E C O M TABLE OF contents The SECOM platform 4 Applications - sections 5 Applications - whole cells 8 Features 9 Integrated workflow 12 Automated overlay ODEMIS - integrated software Specifications 13
Supplementary Figure 1
 Supplementary Figure 1 Technical overview drawing of the Roadrunner goniometer. The goniometer consists of three main components: an inline sample-viewing microscope, a high-precision scanning unit for
Supplementary Figure 1 Technical overview drawing of the Roadrunner goniometer. The goniometer consists of three main components: an inline sample-viewing microscope, a high-precision scanning unit for
The application of spherical aberration correction and focal series restoration to high-resolution images of platinum nanocatalyst particles
 Journal of Physics: Conference Series The application of spherical aberration correction and focal series restoration to high-resolution images of platinum nanocatalyst particles Recent citations - Miguel
Journal of Physics: Conference Series The application of spherical aberration correction and focal series restoration to high-resolution images of platinum nanocatalyst particles Recent citations - Miguel
CTF Correction with IMOD
 CTF Correction with IMOD CTF Correction When microscope is operated in underfocus to produce phase contrast, the contrast is inverted in some spatial frequency ranges 1 We See Only Amplitudes, Not Phases,
CTF Correction with IMOD CTF Correction When microscope is operated in underfocus to produce phase contrast, the contrast is inverted in some spatial frequency ranges 1 We See Only Amplitudes, Not Phases,
MSE 460 TEM Lab 2: Basic Alignment and Operation of Microscope
 MSE 460 TEM Lab 2: Basic Alignment and Operation of Microscope Last updated on 1/8/2018 Jinsong Wu, jinsong-wu@northwestern.edu Aims: The aim of this lab is to familiarize you with basic TEM alignment
MSE 460 TEM Lab 2: Basic Alignment and Operation of Microscope Last updated on 1/8/2018 Jinsong Wu, jinsong-wu@northwestern.edu Aims: The aim of this lab is to familiarize you with basic TEM alignment
Introduction to Electron Microscopy
 Introduction to Electron Microscopy Prof. David Muller, dm24@cornell.edu Rm 274 Clark Hall, 255-4065 Ernst Ruska and Max Knoll built the first electron microscope in 1931 (Nobel Prize to Ruska in 1986)
Introduction to Electron Microscopy Prof. David Muller, dm24@cornell.edu Rm 274 Clark Hall, 255-4065 Ernst Ruska and Max Knoll built the first electron microscope in 1931 (Nobel Prize to Ruska in 1986)
THE BOTTOM LINE I. THE MICROSCOPE
 THE BOTTOM LINE This document is designed to help students focus their attention on basic concepts that are important for understanding the fundamental principles of transmission electron microscopy, biological
THE BOTTOM LINE This document is designed to help students focus their attention on basic concepts that are important for understanding the fundamental principles of transmission electron microscopy, biological
Scanning electron microscope
 Scanning electron microscope 6 th CEMM workshop Maja Koblar, Sc. Eng. Physics Outline The basic principle? What is an electron? Parts of the SEM Electron gun Electromagnetic lenses Apertures Chamber and
Scanning electron microscope 6 th CEMM workshop Maja Koblar, Sc. Eng. Physics Outline The basic principle? What is an electron? Parts of the SEM Electron gun Electromagnetic lenses Apertures Chamber and
Material analysis by infrared mapping: A case study using a multilayer
 Material analysis by infrared mapping: A case study using a multilayer paint sample Application Note Author Dr. Jonah Kirkwood, Dr. John Wilson and Dr. Mustafa Kansiz Agilent Technologies, Inc. Introduction
Material analysis by infrared mapping: A case study using a multilayer paint sample Application Note Author Dr. Jonah Kirkwood, Dr. John Wilson and Dr. Mustafa Kansiz Agilent Technologies, Inc. Introduction
NanoSpective, Inc Progress Drive Suite 137 Orlando, Florida
 TEM Techniques Summary The TEM is an analytical instrument in which a thin membrane (typically < 100nm) is placed in the path of an energetic and highly coherent beam of electrons. Typical operating voltages
TEM Techniques Summary The TEM is an analytical instrument in which a thin membrane (typically < 100nm) is placed in the path of an energetic and highly coherent beam of electrons. Typical operating voltages
Bias errors in PIV: the pixel locking effect revisited.
 Bias errors in PIV: the pixel locking effect revisited. E.F.J. Overmars 1, N.G.W. Warncke, C. Poelma and J. Westerweel 1: Laboratory for Aero & Hydrodynamics, University of Technology, Delft, The Netherlands,
Bias errors in PIV: the pixel locking effect revisited. E.F.J. Overmars 1, N.G.W. Warncke, C. Poelma and J. Westerweel 1: Laboratory for Aero & Hydrodynamics, University of Technology, Delft, The Netherlands,
Operating F20/F30 with SerialEM
 Chen Xu xuchen@brandeis.ede $BrandeisEM: ~emdoc-xml/en_us.iso8859-1/articles/operating-f20-or-f30/article.xml, 1 2013-01-19 01:42:20 xuchen Exp$ This is a quick check list for the Tecnai F20 or Tecnai
Chen Xu xuchen@brandeis.ede $BrandeisEM: ~emdoc-xml/en_us.iso8859-1/articles/operating-f20-or-f30/article.xml, 1 2013-01-19 01:42:20 xuchen Exp$ This is a quick check list for the Tecnai F20 or Tecnai
attocfm I for Surface Quality Inspection NANOSCOPY APPLICATION NOTE M01 RELATED PRODUCTS G
 APPLICATION NOTE M01 attocfm I for Surface Quality Inspection Confocal microscopes work by scanning a tiny light spot on a sample and by measuring the scattered light in the illuminated volume. First,
APPLICATION NOTE M01 attocfm I for Surface Quality Inspection Confocal microscopes work by scanning a tiny light spot on a sample and by measuring the scattered light in the illuminated volume. First,
1.3. Before loading the holder into the TEM, make sure the X tilt is set to zero and the goniometer locked in place (this will make loading easier).
 JEOL 200CX operating procedure Nicholas G. Rudawski ngr@ufl.edu (805) 252-4916 1. Specimen loading 1.1. Unlock the TUMI system. 1.2. Load specimen(s) into the holder. If using the double tilt holder, ensure
JEOL 200CX operating procedure Nicholas G. Rudawski ngr@ufl.edu (805) 252-4916 1. Specimen loading 1.1. Unlock the TUMI system. 1.2. Load specimen(s) into the holder. If using the double tilt holder, ensure
CPSC 4040/6040 Computer Graphics Images. Joshua Levine
 CPSC 4040/6040 Computer Graphics Images Joshua Levine levinej@clemson.edu Lecture 04 Displays and Optics Sept. 1, 2015 Slide Credits: Kenny A. Hunt Don House Torsten Möller Hanspeter Pfister Agenda Open
CPSC 4040/6040 Computer Graphics Images Joshua Levine levinej@clemson.edu Lecture 04 Displays and Optics Sept. 1, 2015 Slide Credits: Kenny A. Hunt Don House Torsten Möller Hanspeter Pfister Agenda Open
AUTOMATED IMAGE ANALYSIS FOR ELECTRON MICROSCOPY SPECIMEN ASSESSMENT
 AUTOMATED IMAGE ANALYSIS FOR ELECTRON MICROSCOPY SPECIMEN ASSESSMENT Nicolas Coudray, Jean-Luc Buessler, Hubert Kihl, Jean-Philippe Urban Laboratoire MIPS, Université de Haute Alsace 4, rue des Frères
AUTOMATED IMAGE ANALYSIS FOR ELECTRON MICROSCOPY SPECIMEN ASSESSMENT Nicolas Coudray, Jean-Luc Buessler, Hubert Kihl, Jean-Philippe Urban Laboratoire MIPS, Université de Haute Alsace 4, rue des Frères
Instructions for the Experiment
 Instructions for the Experiment Excitonic States in Atomically Thin Semiconductors 1. Introduction Alongside with electrical measurements, optical measurements are an indispensable tool for the study of
Instructions for the Experiment Excitonic States in Atomically Thin Semiconductors 1. Introduction Alongside with electrical measurements, optical measurements are an indispensable tool for the study of
FEI Titan Image Corrected STEM
 05/03/16 1 FEI Titan 60-300 Image Corrected STEM Standby Condition HT setting at 300kV, Col. Valves Closed RESET Holder and remove sample. Mag ~ 5-10kX Objective and SA apertures out, C2 aperture at 150µm
05/03/16 1 FEI Titan 60-300 Image Corrected STEM Standby Condition HT setting at 300kV, Col. Valves Closed RESET Holder and remove sample. Mag ~ 5-10kX Objective and SA apertures out, C2 aperture at 150µm
Aberration corrected tilt series restoration
 Journal of Physics: Conference Series Aberration corrected tilt series restoration To cite this article: S Haigh et al 2008 J. Phys.: Conf. Ser. 126 012042 Recent citations - Artefacts in geometric phase
Journal of Physics: Conference Series Aberration corrected tilt series restoration To cite this article: S Haigh et al 2008 J. Phys.: Conf. Ser. 126 012042 Recent citations - Artefacts in geometric phase
Pixel CCD RASNIK. Kevan S Hashemi and James R Bensinger Brandeis University May 1997
 ATLAS Internal Note MUON-No-180 Pixel CCD RASNIK Kevan S Hashemi and James R Bensinger Brandeis University May 1997 Introduction This note compares the performance of the established Video CCD version
ATLAS Internal Note MUON-No-180 Pixel CCD RASNIK Kevan S Hashemi and James R Bensinger Brandeis University May 1997 Introduction This note compares the performance of the established Video CCD version
SCANNING ELECTRON MICROSCOPY AND X-RAY MICROANALYSIS
 SCANNING ELECTRON MICROSCOPY AND X-RAY MICROANALYSIS Robert Edward Lee Electron Microscopy Center Department of Anatomy and Neurobiology Colorado State University P T R Prentice Hall, Englewood Cliffs,
SCANNING ELECTRON MICROSCOPY AND X-RAY MICROANALYSIS Robert Edward Lee Electron Microscopy Center Department of Anatomy and Neurobiology Colorado State University P T R Prentice Hall, Englewood Cliffs,
RENISHAW INVIA RAMAN SPECTROMETER
 STANDARD OPERATING PROCEDURE: RENISHAW INVIA RAMAN SPECTROMETER Purpose of this Instrument: The Renishaw invia Raman Spectrometer is an instrument used to analyze the Raman scattered light from samples
STANDARD OPERATING PROCEDURE: RENISHAW INVIA RAMAN SPECTROMETER Purpose of this Instrument: The Renishaw invia Raman Spectrometer is an instrument used to analyze the Raman scattered light from samples
Compressive Through-focus Imaging
 PIERS ONLINE, VOL. 6, NO. 8, 788 Compressive Through-focus Imaging Oren Mangoubi and Edwin A. Marengo Yale University, USA Northeastern University, USA Abstract Optical sensing and imaging applications
PIERS ONLINE, VOL. 6, NO. 8, 788 Compressive Through-focus Imaging Oren Mangoubi and Edwin A. Marengo Yale University, USA Northeastern University, USA Abstract Optical sensing and imaging applications
Agilent 8700 LDIR Chemical Imaging System. Bringing Clarity and Unprecedented Speed to Chemical Imaging.
 Agilent 8700 LDIR Chemical Imaging System Bringing Clarity and Unprecedented Speed to Chemical Imaging. What if you could save time and achieve better results? The Agilent 8700 Laser Direct Infrared (LDIR)
Agilent 8700 LDIR Chemical Imaging System Bringing Clarity and Unprecedented Speed to Chemical Imaging. What if you could save time and achieve better results? The Agilent 8700 Laser Direct Infrared (LDIR)
Buzz Words (Transmission Electron Microscopy)
 Buzz Words (Transmission Electron Microscopy) Airy disk amplitude contrast angular aperture anticontaminator aperture contrast astigmatism barrel distortion BFEM (Bright Field EM) blind imaging Bragg reflection
Buzz Words (Transmission Electron Microscopy) Airy disk amplitude contrast angular aperture anticontaminator aperture contrast astigmatism barrel distortion BFEM (Bright Field EM) blind imaging Bragg reflection
Diamond X-ray Rocking Curve and Topograph Measurements at CHESS
 Diamond X-ray Rocking Curve and Topograph Measurements at CHESS G. Yang 1, R.T. Jones 2, F. Klein 3 1 Department of Physics and Astronomy, University of Glasgow, Glasgow, UK G12 8QQ. 2 University of Connecticut
Diamond X-ray Rocking Curve and Topograph Measurements at CHESS G. Yang 1, R.T. Jones 2, F. Klein 3 1 Department of Physics and Astronomy, University of Glasgow, Glasgow, UK G12 8QQ. 2 University of Connecticut
1. Specimen Holder Removal, Loading, and Insertion
 OPERATION OF THE PHILIPS CM-200 FEG-TEM When not in use, the CM-200 should be in the MICROSCOPE ON configuration with the HIGH TENSION ON (illuminates green when the high tension is on).. The microscope
OPERATION OF THE PHILIPS CM-200 FEG-TEM When not in use, the CM-200 should be in the MICROSCOPE ON configuration with the HIGH TENSION ON (illuminates green when the high tension is on).. The microscope
Fastest high definition Raman imaging. Fastest Laser Raman Microscope RAMAN
 Fastest high definition Raman imaging Fastest Laser Raman Microscope RAMAN - 11 www.nanophoton.jp Observation A New Generation in Raman Observation RAMAN-11 developed by Nanophoton was newly created by
Fastest high definition Raman imaging Fastest Laser Raman Microscope RAMAN - 11 www.nanophoton.jp Observation A New Generation in Raman Observation RAMAN-11 developed by Nanophoton was newly created by
Agilent Cary 610/620 FTIR microscopes and imaging systems RESOLUTION FOR EVERY APPLICATION
 Agilent Cary 610/620 FTIR microscopes and imaging systems RESOLUTION FOR EVERY APPLICATION AGILENT CARY 610/620 FTIR MICROSCOPES ADVANCING FTIR MICROSCOPY AND IMAGING Agilent s 610/620 FTIR microscopes
Agilent Cary 610/620 FTIR microscopes and imaging systems RESOLUTION FOR EVERY APPLICATION AGILENT CARY 610/620 FTIR MICROSCOPES ADVANCING FTIR MICROSCOPY AND IMAGING Agilent s 610/620 FTIR microscopes
How to choose the optimal microscope/camera combinations
 How to choose the optimal microscope/camera combinations The Practical Matters Anchi Cheng National Resource for Automated Common Mistakes I have money; I will get everything, regardless. I don t have
How to choose the optimal microscope/camera combinations The Practical Matters Anchi Cheng National Resource for Automated Common Mistakes I have money; I will get everything, regardless. I don t have
WITec Alpha 300R Quick Operation Summary October 2018
 WITec Alpha 300R Quick Operation Summary October 2018 This document is frequently updated if you feel information should be added, please indicate that to the facility manager (currently Philip Carubia,
WITec Alpha 300R Quick Operation Summary October 2018 This document is frequently updated if you feel information should be added, please indicate that to the facility manager (currently Philip Carubia,
Nature Protocols: doi: /nprot
 Supplementary Tutorial A total of nine examples illustrating different aspects of data processing referred to in the text are given here. Images for these examples can be downloaded from www.mrc- lmb.cam.ac.uk/harry/imosflm/examples.
Supplementary Tutorial A total of nine examples illustrating different aspects of data processing referred to in the text are given here. Images for these examples can be downloaded from www.mrc- lmb.cam.ac.uk/harry/imosflm/examples.
Be aware that there is no universal notation for the various quantities.
 Fourier Optics v2.4 Ray tracing is limited in its ability to describe optics because it ignores the wave properties of light. Diffraction is needed to explain image spatial resolution and contrast and
Fourier Optics v2.4 Ray tracing is limited in its ability to describe optics because it ignores the wave properties of light. Diffraction is needed to explain image spatial resolution and contrast and
Scanning Electron Microscopy. EMSE-515 F. Ernst
 Scanning Electron Microscopy EMSE-515 F. Ernst 1 2 Scanning Electron Microscopy Max Knoll Manfred von Ardenne Manfred von Ardenne Principle of Scanning Electron Microscopy 3 Principle of Scanning Electron
Scanning Electron Microscopy EMSE-515 F. Ernst 1 2 Scanning Electron Microscopy Max Knoll Manfred von Ardenne Manfred von Ardenne Principle of Scanning Electron Microscopy 3 Principle of Scanning Electron
Diffractogram tableaux by mouse click
 Ultramicroscopy 93 (2002) 77 82 Diffractogram tableaux by mouse click Johannes Zemlin a, Friedrich Zemlin b, * a Lichter felder Ring 123, D-12209 Berlin, Germany b Fritz-Haber-Institut der MPG, Abteilung
Ultramicroscopy 93 (2002) 77 82 Diffractogram tableaux by mouse click Johannes Zemlin a, Friedrich Zemlin b, * a Lichter felder Ring 123, D-12209 Berlin, Germany b Fritz-Haber-Institut der MPG, Abteilung
Confocal Imaging Through Scattering Media with a Volume Holographic Filter
 Confocal Imaging Through Scattering Media with a Volume Holographic Filter Michal Balberg +, George Barbastathis*, Sergio Fantini % and David J. Brady University of Illinois at Urbana-Champaign, Urbana,
Confocal Imaging Through Scattering Media with a Volume Holographic Filter Michal Balberg +, George Barbastathis*, Sergio Fantini % and David J. Brady University of Illinois at Urbana-Champaign, Urbana,
Bringing Answers to the Surface
 3D Bringing Answers to the Surface 1 Expanding the Boundaries of Laser Microscopy Measurements and images you can count on. Every time. LEXT OLS4100 Widely used in quality control, research, and development
3D Bringing Answers to the Surface 1 Expanding the Boundaries of Laser Microscopy Measurements and images you can count on. Every time. LEXT OLS4100 Widely used in quality control, research, and development
NANO 703-Notes. Chapter 9-The Instrument
 1 Chapter 9-The Instrument Illumination (condenser) system Before (above) the sample, the purpose of electron lenses is to form the beam/probe that will illuminate the sample. Our electron source is macroscopic
1 Chapter 9-The Instrument Illumination (condenser) system Before (above) the sample, the purpose of electron lenses is to form the beam/probe that will illuminate the sample. Our electron source is macroscopic
Technical Notes. Integrating Sphere Measurement Part II: Calibration. Introduction. Calibration
 Technical Notes Integrating Sphere Measurement Part II: Calibration This Technical Note is Part II in a three part series examining the proper maintenance and use of integrating sphere light measurement
Technical Notes Integrating Sphere Measurement Part II: Calibration This Technical Note is Part II in a three part series examining the proper maintenance and use of integrating sphere light measurement
Surface Finish Measurement Methods and Instrumentation
 125 years of innovation Surface Finish Measurement Methods and Instrumentation Contents Visual Inspection Surface Finish Comparison Plates Contact Gauges Inductive / Variable Reluctance (INTRA) Piezo Electric
125 years of innovation Surface Finish Measurement Methods and Instrumentation Contents Visual Inspection Surface Finish Comparison Plates Contact Gauges Inductive / Variable Reluctance (INTRA) Piezo Electric
ME 6406 MACHINE VISION. Georgia Institute of Technology
 ME 6406 MACHINE VISION Georgia Institute of Technology Class Information Instructor Professor Kok-Meng Lee MARC 474 Office hours: Tues/Thurs 1:00-2:00 pm kokmeng.lee@me.gatech.edu (404)-894-7402 Class
ME 6406 MACHINE VISION Georgia Institute of Technology Class Information Instructor Professor Kok-Meng Lee MARC 474 Office hours: Tues/Thurs 1:00-2:00 pm kokmeng.lee@me.gatech.edu (404)-894-7402 Class
05/20/14 1. Philips CM200T. Standby Condition
 05/20/14 1 Philips CM200T Standby Condition HT and filament off, HT setting at 200kV. RESET HOLDER, center sample tilt knobs, and remove sample. Mag ~ 5-10kX Objective and SA apertures out, C2 aperture
05/20/14 1 Philips CM200T Standby Condition HT and filament off, HT setting at 200kV. RESET HOLDER, center sample tilt knobs, and remove sample. Mag ~ 5-10kX Objective and SA apertures out, C2 aperture
Nanotechnology in Consumer Products
 Nanotechnology in Consumer Products Advances in Transmission Electron Microscopy Friday, April 21, 2017 October 31, 2014 The webinar will begin at 1pm Eastern Time Click here to watch the webinar recording
Nanotechnology in Consumer Products Advances in Transmission Electron Microscopy Friday, April 21, 2017 October 31, 2014 The webinar will begin at 1pm Eastern Time Click here to watch the webinar recording
Scanning electron microscope
 Scanning electron microscope 5 th CEMM workshop Maja Koblar, Sc. Eng. Physics Outline The basic principle? What is an electron? Parts of the SEM Electron gun Electromagnetic lenses Apertures Detectors
Scanning electron microscope 5 th CEMM workshop Maja Koblar, Sc. Eng. Physics Outline The basic principle? What is an electron? Parts of the SEM Electron gun Electromagnetic lenses Apertures Detectors
Light Microscopy. Upon completion of this lecture, the student should be able to:
 Light Light microscopy is based on the interaction of light and tissue components and can be used to study tissue features. Upon completion of this lecture, the student should be able to: 1- Explain the
Light Light microscopy is based on the interaction of light and tissue components and can be used to study tissue features. Upon completion of this lecture, the student should be able to: 1- Explain the
DualBeam and FIB capability applied to metals research
 DualBeam and FIB capability applied to metals research The values of DualBeam for metals research The availability of Focused Ion Beam (FIB) capacity on a DualBeam has allowed many researchers to open
DualBeam and FIB capability applied to metals research The values of DualBeam for metals research The availability of Focused Ion Beam (FIB) capacity on a DualBeam has allowed many researchers to open
Optical design of a high resolution vision lens
 Optical design of a high resolution vision lens Paul Claassen, optical designer, paul.claassen@sioux.eu Marnix Tas, optical specialist, marnix.tas@sioux.eu Prof L.Beckmann, l.beckmann@hccnet.nl Summary:
Optical design of a high resolution vision lens Paul Claassen, optical designer, paul.claassen@sioux.eu Marnix Tas, optical specialist, marnix.tas@sioux.eu Prof L.Beckmann, l.beckmann@hccnet.nl Summary:
Paul Mooney Gatan, Inc. October 31, 2017
 Paul Mooney Gatan, Inc. October 31, 2017 Leverage Detection Algo Image formation Resolution (Å) Electron-counting cryo-electron microscopy* 4 3.5 3 2.5 *Hong Zhou in: Science, 6/30/2017 and J. General
Paul Mooney Gatan, Inc. October 31, 2017 Leverage Detection Algo Image formation Resolution (Å) Electron-counting cryo-electron microscopy* 4 3.5 3 2.5 *Hong Zhou in: Science, 6/30/2017 and J. General
Large Field of View, High Spatial Resolution, Surface Measurements
 Large Field of View, High Spatial Resolution, Surface Measurements James C. Wyant and Joanna Schmit WYKO Corporation, 2650 E. Elvira Road Tucson, Arizona 85706, USA jcwyant@wyko.com and jschmit@wyko.com
Large Field of View, High Spatial Resolution, Surface Measurements James C. Wyant and Joanna Schmit WYKO Corporation, 2650 E. Elvira Road Tucson, Arizona 85706, USA jcwyant@wyko.com and jschmit@wyko.com
On spatial resolution
 On spatial resolution Introduction How is spatial resolution defined? There are two main approaches in defining local spatial resolution. One method follows distinction criteria of pointlike objects (i.e.
On spatial resolution Introduction How is spatial resolution defined? There are two main approaches in defining local spatial resolution. One method follows distinction criteria of pointlike objects (i.e.
MSE 595T Transmission Electron Microscopy. Laboratory III TEM Imaging - I
 MSE 595T Basic Transmission Electron Microscopy TEM Imaging - I Purpose The purpose of this lab is to: 1. Make fine adjustments to the microscope alignment 2. Obtain a diffraction pattern 3. Obtain an
MSE 595T Basic Transmission Electron Microscopy TEM Imaging - I Purpose The purpose of this lab is to: 1. Make fine adjustments to the microscope alignment 2. Obtain a diffraction pattern 3. Obtain an
Southern African Large Telescope. RSS CCD Geometry
 Southern African Large Telescope RSS CCD Geometry Kenneth Nordsieck University of Wisconsin Document Number: SALT-30AM0011 v 1.0 9 May, 2012 Change History Rev Date Description 1.0 9 May, 2012 Original
Southern African Large Telescope RSS CCD Geometry Kenneth Nordsieck University of Wisconsin Document Number: SALT-30AM0011 v 1.0 9 May, 2012 Change History Rev Date Description 1.0 9 May, 2012 Original
Figure 1 The Raith 150 TWO
 RAITH 150 TWO SOP Figure 1 The Raith 150 TWO LOCATION: Raith 150 TWO room, Lithography area, NanoFab PRIMARY TRAINER: SECONDARY TRAINER: 1. OVERVIEW The Raith 150 TWO is an ultra high resolution, low voltage
RAITH 150 TWO SOP Figure 1 The Raith 150 TWO LOCATION: Raith 150 TWO room, Lithography area, NanoFab PRIMARY TRAINER: SECONDARY TRAINER: 1. OVERVIEW The Raith 150 TWO is an ultra high resolution, low voltage
Fast Laser Raman Microscope RAMAN
 Fast Laser Raman Microscope RAMAN - 11 www.nanophoton.jp Fast Raman Imaging A New Generation of Raman Microscope RAMAN-11 developed by Nanophoton was created by combining confocal laser microscope technology
Fast Laser Raman Microscope RAMAN - 11 www.nanophoton.jp Fast Raman Imaging A New Generation of Raman Microscope RAMAN-11 developed by Nanophoton was created by combining confocal laser microscope technology
Cs-corrector. Felix de Haas
 Cs-corrector. Felix de Haas Content Non corrector systems Lens aberrations and how to minimize? Corrector systems How is it done? Lens aberrations Spherical aberration Astigmatism Coma Chromatic Quality
Cs-corrector. Felix de Haas Content Non corrector systems Lens aberrations and how to minimize? Corrector systems How is it done? Lens aberrations Spherical aberration Astigmatism Coma Chromatic Quality
Fast Laser Raman Microscope RAMAN
 Fast Laser Raman Microscope RAMAN - 11 www.nanophoton.jp Fast Raman Imaging A New Generation of Raman Microscope RAMAN-11 developed by Nanophoton was created by combining confocal laser microscope technology
Fast Laser Raman Microscope RAMAN - 11 www.nanophoton.jp Fast Raman Imaging A New Generation of Raman Microscope RAMAN-11 developed by Nanophoton was created by combining confocal laser microscope technology
