Procedures for Performing Cryoelectron Microscopy on the FEI Sphera Microscope
|
|
|
- Annabella Rogers
- 5 years ago
- Views:
Transcription
1 Procedures for Performing Cryoelectron Microscopy on the FEI Sphera Microscope The procedures given below were written specifically for the FEI Tecnai G 2 Sphera microscope. Modifications will need to be made for other FEI, non- Tecnai microscopes and for microscopes from other manufacturers. It will be assumed that the microscope has a thermionic gun, a Gatan 626 cryotransfer holder, and an intensified TV camera. Procedures for freezing the sample, transferring it into the cold holder, and transferring the cold holder into the microscope are discussed in other tutorials. Words in Bold are either software buttons to be pushed or options to enter 1. Before insertion of the cold holder the anticontaminator/cold finger should be filled with nitrogen, the user should be logged onto the computer system, the filament saturated and a beam obtained. Make sure that the column valves on the microscope are closed before inserting the holder. 2. After inserting the holder wait a minimum of a half an hour for the microscope vacuum to recover. Do not retract the cryoshields on the holder. This assures that any vapor that is inserted into the column with the holder will be removed and not become contamination on the sample. 3. Now perform the necessary gun alignments. These need to be done without a sample in the beam. Open the column valves and pull the holder out until it no longer obstructs the beam but not so far out that the holder is pulled into the airlock. The holder may be maintained in this position by propping a pencil between the holder dewar and the goniometer. a. Remove the objective aperture. b. Perform the necessary gun alignments. c. Read in all previously stored microscope alignments except for the gun alignments. 4. Set the low dose magnification and illumination conditions (Fig. 1). a. Turn on Low Dose on the low dose panel. The Search mode may be set up either in low-magnification, bright-field mode or in defocused diffraction mode. i. Bright-field search mode 1. Hit the Search button 2. Set the magnification to between 1000X and 3000X. This should allow the user to view an entire grid square.
2 Fig. 1. Example low dose settings. The Options flap-out box shows the dose measuring feature in the lower right of the image. 3. Set the illumination conditions (C2 aperture, spot size, beam diameter) to make the beam as dim as possible yet still allow the user to judge the quality of the sample on the large, fluorescent, viewing screen. These settings will, obviously, need to be tweaked when the sample is put in the beam. The aperture in position three of the C2 aperture rod is most often used. 4. Example conditions: 100 µm C2 aperture, 2500X, spot size 4, 100 mm beam diameter would give a dose rate of ~0.1 e - /Å 2 /sec. ii. Defocused diffraction search mode 1. Make sure Low Dose is in Search mode 2. The advantage of this mode is that the objective lens current, which changes at different magnifications, remains constant throughout the procedure. This results in a more stable beam. 3. Choose your preferred C2 aperture and center it. The one in position three is most often used. 2
3 4. Put the Magnification into the M mode (1,500 to 3,100X). 5. Hit the Diffraction button on the right-hand control pad. 6. Turn the Camera Length knob (same knob as magnification in bright field) to around 135mm and spread the beam with the C2 illumination knob. This should give you a view of a majority of a grid square. 7. Select a spot size that is as small as you can get and still be able to see the holes in the carbon film. This will reduce the dose the sample gets while in search mode. This beam diameter and beam settings should not be changed throughout the rest of the low dose session. b. Set up the Exposure mode. i. Go to Exposure mode ii. Set the desired final magnification. This decision in based on the size of the macromolecule, the pixel size at the specimen magnification, the digitization step size of the scanner if the film is to be scanned, and the expected resolution of the final three-dimensional reconstruction. iii. Center and spread the beam until it reaches the edge of the view screen. On the Sphera this diameter is 165 mm. This beam diameter and beam settings should not be changed throughout the rest of the low dose session although they should occasionally be checked to make sure nothing has not moved. iv. Measure the electron dose in the Exposure mode (Fig. 1). Open the flapout box and click on the Options tab. Make sure that 165 mm is 3
4 in the beam diameter box at the bottom of the screen and hit the Measure button. The readout shows the dose in electrons/nm 2. Move the decimal point two positions to the left to get e - /Å 2. For 200 kv electrons the dose rate should be between e - /Å 2 /sec, at 50,000X, and an exposure of 1 second when using film. It is best to keep the value as low as possible but the density on the developed film will be too light if the exposure is much less. Change the C2 spot size to change the beam brightness. v. If you are using film go to the Plate Camera (User) page and set the film camera parameters (Fig. 2). The Hold box should be checked, the camera should be set on Manual rather than on Automatic exposure, and the exposure time should be set to obtain the desired dose based on the measurement. The Film text box is for typing in any information you may want to see printed on the bottom of the film. Make sure you hit the key to enter the information. Also take note that this information will be printed on all of the films until you erase or change it. vi. On the Low Dose page hit the flap-out button and go to the Settings Fig. 3. Choosing the plate film camera in the settings flap-out box in low dose. tab. Set the Use: option for the Exposure mode to use Plate Camera if you are using film or US1000 if you are using the CCD. The CCD camera will have to be on in order to see this option (Fig. 3). If you are using the CCD enter the time of your exposure in the Exposure time (s) 4
5 box in the Exposure tab on the bottom half of the main Low Dose page (Fig. 1). c. Set up the Focus mode. It is best to have the magnification on the focus and exposure modes the same to minimize changes in the objective lens currents. The decision to do this, however, is usually based on if it is possible to do focusing and astigmatism correction at the working magnification. Focus mode is sometimes set as high as 150,000X to accurately visualize any image astigmatism that may be present. If a TV-rate or a CCD camera is present it is possible to use lower Focus mode magnifications to do the corrections. i. The diameter of the beam and spot size settings are not a factor here as long as the beam is not spread so far as to dose the Exposure area. Some microscopists suggest that the spot sizes be set the same in all three modes. ii. Example conditions: At 50,000X a focus distance of 2 µm and angles set at 0 and 180 degrees are sufficient. These values are set Fig. 4. Low magnification view of a grid square. A mixture of dry holes and holes covered with the proper thickness of vitreous ice can be seen. This regular array of holes is a feature in the commercial Quantifoil and C-Flat films. with the Multifunction knobs and the settings put the focus points and the exposure point along the tilt axis of the holder. If commercially-available C-flats ( or Quantifoil ( films are used (Fig. 4), the angles may have to be changed if the focus points fall in adjacent holes rather than on the carbon. 5. Put the holder back into the beam. Minimize potential drift by removing any ice crystals in the cryoholder dewar that form nucleation points and cause bubbling. This is done by taking a cotton-tipped applicator stick and rubbing along all of the inside surfaces of the dewar and the central rod. The crystals then may be blown out of the 5
6 dewar with a device that is made of a rubber stopper through which a length of plastic tubing has been inserted. Put the tubing in the dewar of the holder and hold the stopper against the opening until enough pressure is formed that it blows out some the liquid nitrogen and the ice crystals. Look in the dewar with a flashlight to see if the bubbling in the nitrogen has stopped. It is also best that the level of nitrogen not be high enough to touch the central rod. 6. Go to Search mode. Pull back the cryoshields on the holder and check to see if you have a beam. Find a grid square that is nearly dry or one that can be sacrificed. Your remaining alignments will be done in this area. 7. Adjust the holder Eucentric Height. During microscopy if you ever move a distance of more than a few grid squares recheck this. 8. Go to Direct Alignments and correct the Rotation Center. 9. Place an objective aperture into the beam and center it. Most of the time the aperture in position three is sufficient but if more contrast is desired the aperture in position two may be used. When searching for holes of an appropriate ice thickness it is often useful to alternatively put in and retract the aperture. Areas of extremely thick ice will be too thick to get a beam through, however, if the objective aperture is removed there may be enough brightness to allow for navigation to a thinner region of the grid. 10. Align the Search and Exposure mode positions. This is necessary so that the area that is chosen in Search mode will be the same as the one photographed in Exposure mode. a. Place the beam stop/pointer into the beam and center it on the small circle in the middle of the large fluorescent screen. b. Find an object of interest that will be recognizable at the both the Exposure mode and Search Mode magnifications. Many times this can be a rip in the film or a large chunk of ice such as that in the upper right corner of the grid in Figure 4. Go to Exposure mode and move the object with the goniometer specimen shifts until the object is centered under the tip of the pointer. c. Go to Search Mode. Move the object of interest with the Multifunction X and Y knobs (image shift) until it is under the tip of the pointer. If the Exposure and Search Modes are closely aligned this should be easy otherwise this may need to be repeated until the object is aligned in both modes. d. Find a new object of interest and check the positioning in both modes again. Make adjustments with the specimen shift while in Exposure Mode and with the Multifunction X and Y knobs while in Search Mode. 6
7 e. If you are doing your searching in bright-field mode defocus the objective lens several hundred microns and then, if the object moves, bring it back into position with the Multifunction X and Y knobs. This increases the contrast in the search mode and makes it easier to recognize the sample in the ice. This will not be necessary if the defocused diffraction mode is chosen for searching. 11. In Search mode look across the grid for suitable areas of ice thickness and sample concentration for data collection (Fig. 4). Judging the appropriate thickness of ice will become more accurate with experience. a. When a good area is located that has the appropriate ice thickness go to one of the corners of the grid square and recheck the eucentric height adjustment. b. A few techniques may be used to determine if there is sample in the ice-covered holes and if the sample is properly dispersed. i. If a bottom-mounted, intensified, TV-rate camera is available the extra magnification obtained by the difference in the physical mounting height of the camera compared with that of the fluorescent screen may be enough to judge the quality of the preparation. ii. Another method is to temporarily increase the magnification of the Search mode until beam damage causes bubbling in the sample. This usually occurs first in the biological sample which makes it possible to distinguish the sample from the background. This, obviously, destroys this area and makes it unsuitable for photography. Checking a few areas should result in a general idea of the sample distribution. iii. If a CCD camera is present a few images may be recorded to check the sample. 12. When an appropriate hole is found in search mode, center it under the pointer and go to Focus mode. If the first Focus mode area falls on an undesirable area such as a dry hole select the second Focus mode area. Focus and stigmate the image. This may be done with the binoculars on the fluorescent screen, with an intensified, TV-rate camera or by calculating live FFTs from images collected with the CCD camera. In Focus mode it is permissible to change the size of the beam with the Intensity knob without affecting the beam in the other modes. This makes it much easier to focus the image. 13. Focus the image to true focus and hit the Reset Defocus button. This button is usually one of the options of the L and R user buttons that are on the inside edge of the control pads. The Reset Defocus button zeroes out the focus value but it does not affect the actual focus 7
8 setting of the objective lens. It is then possible to defocus the image to the desired amount and view the value of the defocus in the display box at the bottom of the screen. 14. Hit the Expose button on the left control pad (not the Exposure Mode in Low Dose). If you are using film you will hear a film plate being put into position. The shutter will open for the set amount of time and then the film plate will be placed into the take-up box. If you are using the CCD, the software will put the CCD camera into the beam and continue similarly as that of film. If you would like a preview of what you recorded on film, hit the beam Blank, then go to Exposure mode. When the beam is in position quickly remove the beam Blank and take a CCD exposure manually with the Digital Micrograph software. After the image has been recorded put the beam Blank back on and return to Focus mode. Obviously, this image will not be suitable for processing but it will help you to pre-judge the quality of your recorded film image. 15. After the exposure is complete it is a good idea to occasionally check the Exposure mode to see if the beam conditions have remained constant and that the area that was chosen to be photographed is still centered. 16. Go back to Search Mode. Note the areas of the grid square that were burned due to focusing and photographing. Note the proximity of the two (or three) burn spots to make sure that your focus points are not dosing the areas you are recording. It is a good idea to always focus your image in an area where data has already been recorded to maximize the amount of data that can be collected from one grid square. If you are using C-flat or Quantifoil grids you may need to adjust the focus distance and angle in Focus Mode if your focus points end up in adjacent, good-ice holes. 17. Systematically move across the grid to make sure that you are always moving onto a new area and not onto one that has already been damaged by the beam. The use of the specimen position display in Stage 2, and with Tracks turned on, should greatly help you decide if you have been in an area before. 18. If you are collecting data with the CCD camera it is a good practice to save your images as you collect them and not have more than a few images open on the screen at one time. The computer does not have the capacity to run the microscope and keep many images open at the same time. Too many images may cause problems with the microscope operating system. It is easiest to name your images with the automatic functions in Digital Micrograph. a. Go to File/Global Info and click on the option Save Numbered in the left hand panel (Fig. 5). 8
9 b. Create or pick a folder in the File Directory. One way to name folders and files is to use a combination of the date and the sample name. For example: BobVirus. The date, September 10, 2008 is written the way it is so that folders are organized, and can be sorted, chronologically. c. Check the radio button Build Using String and then add some text to name your files. A good practice here is to use the same file name that you used for your folder. d. Set Next Index to 1. e. Under the File Content and Format area click on the radio Fig. 5. The Save Numbered option in the Global Info box in Digital Micrograph. button Save Image As and in the dropdown box choose Gatan Format. The reason for this is explained in f. Hit OK. g. To save an image go to File/Save Numbered or just hit CTRL- Y. Close the image by hitting CTRL-W. h. The DM3 format has its advantages but it can only be read by a few programs. Most users convert their DM3s into Tiffs and this can be done in batch mode. Click on File/Batch Convert and pick the folder where you stored your images by clicking on Browse. i. In the File Content and Format area choose the radio button Save Display As and choose Tiff Format as the option in the drop down box. Hit OK to continue. If any of your images are still open they will not be converted. 19. If you are using film and you have completed your data collection remove and replace the film camera. Make sure you hit the film stock reset button so the next user knows there is available film in the microscope. Since the film has been underexposed for low dose 9
10 conditions it must be developed under low-dose conditions. Check out for specifics on developing the film. 10
FEI Tecnai G 2 F20 Operating Procedures
 FEI Tecnai G 2 F20 Operating Procedures 1. Startup (1) Sign-up in the microscope log-sheet. Please ensure you have written an account number for billing. (2) Log in to the computer: Login to your account
FEI Tecnai G 2 F20 Operating Procedures 1. Startup (1) Sign-up in the microscope log-sheet. Please ensure you have written an account number for billing. (2) Log in to the computer: Login to your account
1.2. Make sure the viewing screen is covered (exposure to liquid N 2 may cause it to crack).
 FEI Tecnai F20 S/TEM: imaging in TEM mode Nicholas G. Rudawski ngr@ufl.edu (805) 252-4916 (352) 392-3077 Last updated: 01/21/18 1. Filling the cold trap (if needed) 1.1. Prior to use, the cold trap needs
FEI Tecnai F20 S/TEM: imaging in TEM mode Nicholas G. Rudawski ngr@ufl.edu (805) 252-4916 (352) 392-3077 Last updated: 01/21/18 1. Filling the cold trap (if needed) 1.1. Prior to use, the cold trap needs
Instructions for Tecnai a brief start up manual
 Instructions for Tecnai a brief start up manual Version 3.0, 8.12.2015 Manual of Tecnai 12 transmission electron microscope located at Aalto University's Nanomicroscopy Center. More information of Nanomicroscopy
Instructions for Tecnai a brief start up manual Version 3.0, 8.12.2015 Manual of Tecnai 12 transmission electron microscope located at Aalto University's Nanomicroscopy Center. More information of Nanomicroscopy
Full-screen mode Popup controls. Overview of the microscope user interface, TEM User Interface and TIA on the left and EDS on the right
 Quick Guide to Operating FEI Titan Themis G2 200 (S)TEM: TEM mode Susheng Tan Nanoscale Fabrication and Characterization Facility, University of Pittsburgh Office: M104/B01 Benedum Hall, 412-383-5978,
Quick Guide to Operating FEI Titan Themis G2 200 (S)TEM: TEM mode Susheng Tan Nanoscale Fabrication and Characterization Facility, University of Pittsburgh Office: M104/B01 Benedum Hall, 412-383-5978,
Operating the Hitachi 7100 Transmission Electron Microscope Electron Microscopy Core, University of Utah
 Operating the Hitachi 7100 Transmission Electron Microscope Electron Microscopy Core, University of Utah Follow the procedures below when you use the Hitachi 7100 TEM. Starting Session 1. Turn on the cold
Operating the Hitachi 7100 Transmission Electron Microscope Electron Microscopy Core, University of Utah Follow the procedures below when you use the Hitachi 7100 TEM. Starting Session 1. Turn on the cold
1. Specimen Holder Removal, Loading, and Insertion
 OPERATION OF THE PHILIPS CM-200 FEG-TEM When not in use, the CM-200 should be in the MICROSCOPE ON configuration with the HIGH TENSION ON (illuminates green when the high tension is on).. The microscope
OPERATION OF THE PHILIPS CM-200 FEG-TEM When not in use, the CM-200 should be in the MICROSCOPE ON configuration with the HIGH TENSION ON (illuminates green when the high tension is on).. The microscope
Basic Users Manual for Tecnai-F20 TEM
 Basic Users Manual for Tecnai-F20 TEM NB: This document contains my personal notes on the operating procedure of the Tecnai F20 and may be used as a rough guide for those new to the microscope. It may
Basic Users Manual for Tecnai-F20 TEM NB: This document contains my personal notes on the operating procedure of the Tecnai F20 and may be used as a rough guide for those new to the microscope. It may
Please follow these instructions for use of the Philips CM100 TEM. Adopted from website below.
 Please follow these instructions for use of the Philips CM100 TEM. Adopted from website below. http://staff.washington.edu/wpchan/if/cm100_inst.shtml Instructions for the Philips CM100 TEM and peripherals
Please follow these instructions for use of the Philips CM100 TEM. Adopted from website below. http://staff.washington.edu/wpchan/if/cm100_inst.shtml Instructions for the Philips CM100 TEM and peripherals
2 How to operate the microscope/obtain an image
 Morgagni Operating Instructions 50079 010912 2-1 2 ow to operate the microscope/obtain an image 2.1 Starting the microscope 2.1.1 Starting the microscope with several manually-operated steps 1. Turn on
Morgagni Operating Instructions 50079 010912 2-1 2 ow to operate the microscope/obtain an image 2.1 Starting the microscope 2.1.1 Starting the microscope with several manually-operated steps 1. Turn on
MSE 460 TEM Lab 2: Basic Alignment and Operation of Microscope
 MSE 460 TEM Lab 2: Basic Alignment and Operation of Microscope Last updated on 1/8/2018 Jinsong Wu, jinsong-wu@northwestern.edu Aims: The aim of this lab is to familiarize you with basic TEM alignment
MSE 460 TEM Lab 2: Basic Alignment and Operation of Microscope Last updated on 1/8/2018 Jinsong Wu, jinsong-wu@northwestern.edu Aims: The aim of this lab is to familiarize you with basic TEM alignment
1.3. Before loading the holder into the TEM, make sure the X tilt is set to zero and the goniometer locked in place (this will make loading easier).
 JEOL 200CX operating procedure Nicholas G. Rudawski ngr@ufl.edu (805) 252-4916 1. Specimen loading 1.1. Unlock the TUMI system. 1.2. Load specimen(s) into the holder. If using the double tilt holder, ensure
JEOL 200CX operating procedure Nicholas G. Rudawski ngr@ufl.edu (805) 252-4916 1. Specimen loading 1.1. Unlock the TUMI system. 1.2. Load specimen(s) into the holder. If using the double tilt holder, ensure
This document assumes the user is already familiar with basic operation of the instrument in TEM mode and use of the Microscope Control interface.
 FEI Tecnai F20 S/TEM: imaging in STEM mode Nicholas G. Rudawski ngr@ufl.edu (805) 252-4916 (352) 392-3077 Last updated: 05/10/18 This document assumes the user is already familiar with basic operation
FEI Tecnai F20 S/TEM: imaging in STEM mode Nicholas G. Rudawski ngr@ufl.edu (805) 252-4916 (352) 392-3077 Last updated: 05/10/18 This document assumes the user is already familiar with basic operation
JEOL JEM-1400 Transmission Electron Microscope Operating Instructions
 JEOL JEM-1400 Transmission Electron Microscope Operating Instructions Anti-contamination device Objective aperture Objective aperture translation knobs Specimen holder Pump/air switch Left hand control
JEOL JEM-1400 Transmission Electron Microscope Operating Instructions Anti-contamination device Objective aperture Objective aperture translation knobs Specimen holder Pump/air switch Left hand control
Protective Equipment Nitrile gloves for handling sample holder and safety glasses for filling liquid nitrogen dewar.
 Emergency Information: 1. Medical Emergencies: Contact 911 and McGill Security 514.398.3000 2. Leave TEM as is. Do NOT shut down the vacuum system. 3. If possible, turn off High Tension and Close Column
Emergency Information: 1. Medical Emergencies: Contact 911 and McGill Security 514.398.3000 2. Leave TEM as is. Do NOT shut down the vacuum system. 3. If possible, turn off High Tension and Close Column
2. Raise HT to 200kVby following the procedure explained in 1.6.
 JEOL 2100 MANUAL Quick check list 1. If needed, fill the reservoir with LN2 2. Raise HT to 200kVby following the procedure explained in 1.6. 3. Insert specimen holder into TEM (Insert holder in airlock,
JEOL 2100 MANUAL Quick check list 1. If needed, fill the reservoir with LN2 2. Raise HT to 200kVby following the procedure explained in 1.6. 3. Insert specimen holder into TEM (Insert holder in airlock,
This document assumes the user is already familiar with basic operation of the instrument in TEM mode and use of the digital camera.
 FEI Tecnai F20 S/TEM: acquiring diffraction patterns Nicholas G. Rudawski ngr@ufl.edu (805) 252-4916 (352) 392-3077 Last updated: 10/18/17 This document assumes the user is already familiar with basic
FEI Tecnai F20 S/TEM: acquiring diffraction patterns Nicholas G. Rudawski ngr@ufl.edu (805) 252-4916 (352) 392-3077 Last updated: 10/18/17 This document assumes the user is already familiar with basic
FEI Titan Image Corrected STEM
 05/03/16 1 FEI Titan 60-300 Image Corrected STEM Standby Condition HT setting at 300kV, Col. Valves Closed RESET Holder and remove sample. Mag ~ 5-10kX Objective and SA apertures out, C2 aperture at 150µm
05/03/16 1 FEI Titan 60-300 Image Corrected STEM Standby Condition HT setting at 300kV, Col. Valves Closed RESET Holder and remove sample. Mag ~ 5-10kX Objective and SA apertures out, C2 aperture at 150µm
Jeol JEM Responsible personell: Endy ( ) Online booking is compulsory!
 Jeol JEM 1230 Responsible personell: Endy (45279377) Online booking is compulsory! After training you will have access to working alone on the instrument. All insertion of samples is done by responsible
Jeol JEM 1230 Responsible personell: Endy (45279377) Online booking is compulsory! After training you will have access to working alone on the instrument. All insertion of samples is done by responsible
Tecnai T12 Operating Procedures
 Tecnai T12 Operating Procedures I. Initial Procedures 1 II. Accelerating Voltage 3 III. Specimen Loading and Holder Insertion/Removal 3 IV. Emission Current 7 V. Alignment 7 VI. Camera Control and Imaging
Tecnai T12 Operating Procedures I. Initial Procedures 1 II. Accelerating Voltage 3 III. Specimen Loading and Holder Insertion/Removal 3 IV. Emission Current 7 V. Alignment 7 VI. Camera Control and Imaging
Operating F20/F30 with SerialEM
 Chen Xu xuchen@brandeis.ede $BrandeisEM: ~emdoc-xml/en_us.iso8859-1/articles/operating-f20-or-f30/article.xml, 1 2013-01-19 01:42:20 xuchen Exp$ This is a quick check list for the Tecnai F20 or Tecnai
Chen Xu xuchen@brandeis.ede $BrandeisEM: ~emdoc-xml/en_us.iso8859-1/articles/operating-f20-or-f30/article.xml, 1 2013-01-19 01:42:20 xuchen Exp$ This is a quick check list for the Tecnai F20 or Tecnai
1. Preliminary sample preparation
 FEI Helios NanoLab 600 standard operating procedure Nicholas G. Rudawski ngr@ufl.edu (352) 392 3077 (office) (805) 252-4916 (cell) Last updated: 03/02/18 What this document provides: an overview of basic
FEI Helios NanoLab 600 standard operating procedure Nicholas G. Rudawski ngr@ufl.edu (352) 392 3077 (office) (805) 252-4916 (cell) Last updated: 03/02/18 What this document provides: an overview of basic
05/20/14 1. Philips CM200T. Standby Condition
 05/20/14 1 Philips CM200T Standby Condition HT and filament off, HT setting at 200kV. RESET HOLDER, center sample tilt knobs, and remove sample. Mag ~ 5-10kX Objective and SA apertures out, C2 aperture
05/20/14 1 Philips CM200T Standby Condition HT and filament off, HT setting at 200kV. RESET HOLDER, center sample tilt knobs, and remove sample. Mag ~ 5-10kX Objective and SA apertures out, C2 aperture
Using the Hitachi 3400-N VP-SEM
 Using the Hitachi 3400-N VP-SEM Opening the Chamber to Load Specimens (This may also be done later using the software) 1. Click the AIR button on the front of the machine: 2. Wait a few minutes until you
Using the Hitachi 3400-N VP-SEM Opening the Chamber to Load Specimens (This may also be done later using the software) 1. Click the AIR button on the front of the machine: 2. Wait a few minutes until you
FEI Falcon Direct Electron Detector. Best Practice Document
 FEI Falcon Direct Electron Detector Best Practice Document 2 1. Introduction FEI Falcon Direct Electron Detector Best Practice Application Guide The FEI Falcon Detector is based on direct electron detection
FEI Falcon Direct Electron Detector Best Practice Document 2 1. Introduction FEI Falcon Direct Electron Detector Best Practice Application Guide The FEI Falcon Detector is based on direct electron detection
JEOL 6500 User Manual
 LOG IN to your session on the computer to the left of the microscope. Starting Conditions 1. Press Ctrl-Alt-Del and log on to the microscope computer. Click on JEOL PC SEM 6500 icon. Click yes if message
LOG IN to your session on the computer to the left of the microscope. Starting Conditions 1. Press Ctrl-Alt-Del and log on to the microscope computer. Click on JEOL PC SEM 6500 icon. Click yes if message
1.1. In regular TEM imaging mode, find a region of interest and set it at eucentric height.
 JEOL 2010F operating procedure Covers operation in STEM mode (See separate procedures for operation in TEM mode and operation of EDS system) Nicholas G. Rudawski ngr@ufl.edu (805) 252-4916 NOTE: this operating
JEOL 2010F operating procedure Covers operation in STEM mode (See separate procedures for operation in TEM mode and operation of EDS system) Nicholas G. Rudawski ngr@ufl.edu (805) 252-4916 NOTE: this operating
User Operation of JEOL 1200 EX II
 **Log onto Computer** Open item program Start Up Procedure User Operation of JEOL 1200 EX II 1. If scope is not running, locate an electron microscopy technician (EMT) to find out why not. 2. Turn up brightness
**Log onto Computer** Open item program Start Up Procedure User Operation of JEOL 1200 EX II 1. If scope is not running, locate an electron microscopy technician (EMT) to find out why not. 2. Turn up brightness
OPERATION OF THE HITACHI S-450 SCANNING ELECTRON MICROSCOPE. by Doug Bray Department of Biological Sciences University of Lethbridge
 OPERATION OF THE HITACHI S-450 SCANNING ELECTRON MICROSCOPE by Doug Bray Department of Biological Sciences University of Lethbridge Revised September, 2000 Note: The terms in bold in this document represent
OPERATION OF THE HITACHI S-450 SCANNING ELECTRON MICROSCOPE by Doug Bray Department of Biological Sciences University of Lethbridge Revised September, 2000 Note: The terms in bold in this document represent
XTEM. --Software for Complex Transmission Electron Microscopy. Version 1.0
 XTEM --Software for Complex Transmission Electron Microscopy Version 1.0 1. Introduction XTEM is the software for complex microscopy on JEOL 3100 electron microscopes. The XTEM software consists of a suite
XTEM --Software for Complex Transmission Electron Microscopy Version 1.0 1. Introduction XTEM is the software for complex microscopy on JEOL 3100 electron microscopes. The XTEM software consists of a suite
LEO 912 TEM Short Manual. Prepared/copyrighted by RH Berg Danforth Plant Science Center
 LEO 912 TEM Short Manual Prepared/copyrighted by RH Berg Danforth Plant Science Center Specimen holder [1] Never touch the holder (outside of the O-ring, double-headed arrow) because finger oils will contaminate
LEO 912 TEM Short Manual Prepared/copyrighted by RH Berg Danforth Plant Science Center Specimen holder [1] Never touch the holder (outside of the O-ring, double-headed arrow) because finger oils will contaminate
JEOL 6700 User Manual 05/18/2009
 JEOL 6700 User Manual 05/18/2009 LOG IN to your session on the computer to the right of the microscope. Starting Conditions 1. Click the button and read the Penning Gauge to ensure that the microscope
JEOL 6700 User Manual 05/18/2009 LOG IN to your session on the computer to the right of the microscope. Starting Conditions 1. Click the button and read the Penning Gauge to ensure that the microscope
Scanning Electron Microscope FEI INSPECT F50. Step by step operation manual
 Scanning Electron Microscope FEI INSPECT F50 Step by step operation manual Scanning Electron Microscope, FEI Inspect F50 FE-SEM-F Observation Flow Saving Data And Analysis Specimen preparation Error check
Scanning Electron Microscope FEI INSPECT F50 Step by step operation manual Scanning Electron Microscope, FEI Inspect F50 FE-SEM-F Observation Flow Saving Data And Analysis Specimen preparation Error check
Last updated 6/12/18. F20 User Manual at the Simons Electron Microscopy Center
 F20 User Manual at the Simons Electron Microscopy Center 1 Table of Contents F20 Information Sheet 2 F20 User Guide (starting your session) 3 F20 User Guide (ending your session) 5 Cryo Screening with
F20 User Manual at the Simons Electron Microscopy Center 1 Table of Contents F20 Information Sheet 2 F20 User Guide (starting your session) 3 F20 User Guide (ending your session) 5 Cryo Screening with
Operating Checklist for using the Scanning Electron Microscope, JEOL JSM 6400.
 Smith College August 2005 Operating Checklist for using the Scanning Electron Microscope, JEOL JSM 6400. CONTENT, page no. Pre-Check, 1 Specimen Insertion, 1 Startup, 2 Filament Saturation, 2 Beam Alignment,
Smith College August 2005 Operating Checklist for using the Scanning Electron Microscope, JEOL JSM 6400. CONTENT, page no. Pre-Check, 1 Specimen Insertion, 1 Startup, 2 Filament Saturation, 2 Beam Alignment,
Standard Operating Procedure for the Amray 1810 Scanning Electron Microscope Version: 29 NOVEMBER 2014
 Standard Operating Procedure for the Amray 1810 Scanning Electron Microscope Version: 29 NOVEMBER 2014 1. Utility Requirements a. System power is supplied by two 120 VAC/20 A circuits. When doing maintenance
Standard Operating Procedure for the Amray 1810 Scanning Electron Microscope Version: 29 NOVEMBER 2014 1. Utility Requirements a. System power is supplied by two 120 VAC/20 A circuits. When doing maintenance
RAITH e-line OPERATING INSTRUCTIONS
 RAITH e-line OPERATING INSTRUCTIONS 1) LOADING A SAMPLE a. Start the system i. On the Column PC (Right side monitor [R]), select the SmartSEM icon to on the desktop to begin the column software. ii. On
RAITH e-line OPERATING INSTRUCTIONS 1) LOADING A SAMPLE a. Start the system i. On the Column PC (Right side monitor [R]), select the SmartSEM icon to on the desktop to begin the column software. ii. On
Transmission Electron Microscopy 9. The Instrument. Outline
 Transmission Electron Microscopy 9. The Instrument EMA 6518 Spring 2009 02/25/09 Outline The Illumination System The Objective Lens and Stage Forming Diffraction Patterns and Images Alignment and Stigmation
Transmission Electron Microscopy 9. The Instrument EMA 6518 Spring 2009 02/25/09 Outline The Illumination System The Objective Lens and Stage Forming Diffraction Patterns and Images Alignment and Stigmation
CM20 USER GUIDE. Duncan Alexander, CIME 2010
 CM20 USER GUIDE Duncan Alexander, CIME 2010 CM20 START UP AND CHECK LIST 2 SPECIMEN EXCHANGE 5 - REMOVING SAMPLE HOLDER 6 - INSERTING SAMPLE HOLDER 7 TURNING ON HT 8 STARTING THE FILAMENT 9 GUN TILT ALIGNMENT
CM20 USER GUIDE Duncan Alexander, CIME 2010 CM20 START UP AND CHECK LIST 2 SPECIMEN EXCHANGE 5 - REMOVING SAMPLE HOLDER 6 - INSERTING SAMPLE HOLDER 7 TURNING ON HT 8 STARTING THE FILAMENT 9 GUN TILT ALIGNMENT
Cryo-Electron Microscopy of Viruses
 Blockkurs Biophysic and Structural Biology 2013 Praktikumsversuch at C-CINA Cryo-Electron Microscopy of Viruses In this practical we will compare electron microscopy of negatively stained and frozen-hydrated
Blockkurs Biophysic and Structural Biology 2013 Praktikumsversuch at C-CINA Cryo-Electron Microscopy of Viruses In this practical we will compare electron microscopy of negatively stained and frozen-hydrated
JSM 6060 LV SCANNING ELECTRON MICROSCOPE STANDARD OPERATING PROCEDURES
 JSM 6060 LV SCANNING ELECTRON MICROSCOPE STANDARD OPERATING PROCEDURES RULES All users must go through a series of standard operation procedure training. For more information contact: Longlong Liao Teaching
JSM 6060 LV SCANNING ELECTRON MICROSCOPE STANDARD OPERATING PROCEDURES RULES All users must go through a series of standard operation procedure training. For more information contact: Longlong Liao Teaching
Basic Operating Instructions for Strata Dual Beam 235 FIB/SEM
 Basic Operating Instructions for Strata Dual Beam 235 FIB/SEM Warning Always adjust your specimen height before closing the chamber door to make sure your specimen will not hit the bottom of the lens;
Basic Operating Instructions for Strata Dual Beam 235 FIB/SEM Warning Always adjust your specimen height before closing the chamber door to make sure your specimen will not hit the bottom of the lens;
JEOL 2010 FasTEM & DigitalMicrograph User's Guide
 JEOL 2010 FasTEM & DigitalMicrograph User's Guide Electron Microscopy Laboratory Instititute of Materials Science University of Connecticut The purpose of this manual is to remind you of the essential
JEOL 2010 FasTEM & DigitalMicrograph User's Guide Electron Microscopy Laboratory Instititute of Materials Science University of Connecticut The purpose of this manual is to remind you of the essential
Phase plates for cryo-em
 Max Planck Institute of Biochemistry Martinsried, Germany MAX PLANCK SOCIETY Phase plates for cryo-em Rado Danev Max Planck Institute of Biochemistry, Martinsried, Germany. EMBO course 2017, London, UK
Max Planck Institute of Biochemistry Martinsried, Germany MAX PLANCK SOCIETY Phase plates for cryo-em Rado Danev Max Planck Institute of Biochemistry, Martinsried, Germany. EMBO course 2017, London, UK
CAPTURING IMAGES ON THE HIGH-MAGNIFICATION MICROSCOPE
 University of Virginia ITC Academic Computing Health Sciences CAPTURING IMAGES ON THE HIGH-MAGNIFICATION MICROSCOPE Introduction The Olympus BH-2 microscope in ACHS s microscope lab has objectives from
University of Virginia ITC Academic Computing Health Sciences CAPTURING IMAGES ON THE HIGH-MAGNIFICATION MICROSCOPE Introduction The Olympus BH-2 microscope in ACHS s microscope lab has objectives from
The user should already be familiar with operation of the instrument in STEM mode, use of the Microscope Control interface, and TIA.
 FEI Tecnai F20 S/TEM: EDS system operation Nicholas G. Rudawski ngr@ufl.edu (805) 252-4916 (352) 392-3077 Last updated: 01/22/18 The user should already be familiar with operation of the instrument in
FEI Tecnai F20 S/TEM: EDS system operation Nicholas G. Rudawski ngr@ufl.edu (805) 252-4916 (352) 392-3077 Last updated: 01/22/18 The user should already be familiar with operation of the instrument in
MSE 595T Transmission Electron Microscopy. Laboratory III TEM Imaging - I
 MSE 595T Basic Transmission Electron Microscopy TEM Imaging - I Purpose The purpose of this lab is to: 1. Make fine adjustments to the microscope alignment 2. Obtain a diffraction pattern 3. Obtain an
MSE 595T Basic Transmission Electron Microscopy TEM Imaging - I Purpose The purpose of this lab is to: 1. Make fine adjustments to the microscope alignment 2. Obtain a diffraction pattern 3. Obtain an
1.1. Log on to the TUMI system (you cannot proceed further until this is done).
 FEI DB235 SEM mode operation Nicholas G. Rudawski ngr@ufl.edu (805) 252-4916 1. Sample loading 1.1. Log on to the TUMI system (you cannot proceed further until this is done). 1.2. The FIB software (xp)
FEI DB235 SEM mode operation Nicholas G. Rudawski ngr@ufl.edu (805) 252-4916 1. Sample loading 1.1. Log on to the TUMI system (you cannot proceed further until this is done). 1.2. The FIB software (xp)
Brightfield Microscopy and Image Acquisition on Spotcam1. by Ryan Taylor/Nancy Kleene Last modified 10/02/05 by Birgit Ehmer
 Brightfield Microscopy and Image Acquisition on Spotcam1 by Ryan Taylor/Nancy Kleene Last modified 10/02/05 by Birgit Ehmer Log onto the computer. Enter your username and password to log onto the server.
Brightfield Microscopy and Image Acquisition on Spotcam1 by Ryan Taylor/Nancy Kleene Last modified 10/02/05 by Birgit Ehmer Log onto the computer. Enter your username and password to log onto the server.
Zeiss LSM 880 Protocol
 Zeiss LSM 880 Protocol 1) System Startup Please note put sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for unused time.
Zeiss LSM 880 Protocol 1) System Startup Please note put sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for unused time.
Introduction: Why electrons?
 Introduction: Why electrons? 1 Radiations Visible light X-rays Electrons Neutrons Advantages Not very damaging Easily focused Eye wonderful detector Small wavelength (Angstroms) Good penetration Small
Introduction: Why electrons? 1 Radiations Visible light X-rays Electrons Neutrons Advantages Not very damaging Easily focused Eye wonderful detector Small wavelength (Angstroms) Good penetration Small
STANDARD OPERATING PROCEDURE: JEOL TEM-2100
 STANDARD OPERATING PROCEDURE: JEOL TEM-2100 Purpose of this Instrument: Essential tool for structural characterization of natural or synthesized nanostructures. Location: WVU - Engineering Sciences Building
STANDARD OPERATING PROCEDURE: JEOL TEM-2100 Purpose of this Instrument: Essential tool for structural characterization of natural or synthesized nanostructures. Location: WVU - Engineering Sciences Building
Zeiss LSM 780 Protocol
 Zeiss LSM 780 Protocol 1) System Startup F Please note the sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for unused time.
Zeiss LSM 780 Protocol 1) System Startup F Please note the sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for unused time.
Operating Checklist for using the Scanning Electron. Microscope, JEOL JSM 6400.
 Smith College August 2009 Operating Checklist for using the Scanning Electron Microscope, JEOL JSM 6400. CONTENT, page no. Pre-Check 1 Startup 1 Specimen Insertion 2 Filament Saturation 2 Beam Alignment
Smith College August 2009 Operating Checklist for using the Scanning Electron Microscope, JEOL JSM 6400. CONTENT, page no. Pre-Check 1 Startup 1 Specimen Insertion 2 Filament Saturation 2 Beam Alignment
Zeiss AxioImager.Z2 Brightfield Protocol
 Zeiss AxioImager.Z2 Brightfield Protocol 1) System Startup Please note put sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge
Zeiss AxioImager.Z2 Brightfield Protocol 1) System Startup Please note put sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge
LowDose for JEOL. --Software LowDose and Advanced Data Acquisition. Version 1.1
 LowDose for JEOL --Software LowDose and Advanced Data Acquisition Version 1.1 1. Introduction This is the LowDose software for the user of JEOL electron microscopes. The LowDose software is a Plug-In for
LowDose for JEOL --Software LowDose and Advanced Data Acquisition Version 1.1 1. Introduction This is the LowDose software for the user of JEOL electron microscopes. The LowDose software is a Plug-In for
SAMUEL ROBERTS NOBLE ELECTRON MICROSCOPY LABORATORY. Operating Procedures for the Zeiss 9 S-2. Transmission Electron Microscope
 1 SAMUEL ROBERTS NOBLE ELECTRON MICROSCOPY LABORATORY Operating Procedures for the Zeiss 9 S-2 Transmission Electron Microscope Prepared by Dr. Scott D. Russell Department of Botany and Microbiology September,
1 SAMUEL ROBERTS NOBLE ELECTRON MICROSCOPY LABORATORY Operating Procedures for the Zeiss 9 S-2 Transmission Electron Microscope Prepared by Dr. Scott D. Russell Department of Botany and Microbiology September,
STEM alignment procedures
 STEM alignment procedures Step 1. ASID alignment mode 1. Write down STD for TEM, and then open the ASID control window from dialogue. Also, start Simple imager viewer program on the Desktop. 2. Click on
STEM alignment procedures Step 1. ASID alignment mode 1. Write down STD for TEM, and then open the ASID control window from dialogue. Also, start Simple imager viewer program on the Desktop. 2. Click on
Check that the pneumatic hose is disconnected!!!! (unless your using the BSE detector, of course)
 JEOL 7000F BASIC OPERATING INSTRUCTIONS-Ver.-2.0 Note: This is minimal operation checklist and does not replace the other reference manuals. Read the manual for Specimen Exchange (JEOL 7000 Specimen Exchange
JEOL 7000F BASIC OPERATING INSTRUCTIONS-Ver.-2.0 Note: This is minimal operation checklist and does not replace the other reference manuals. Read the manual for Specimen Exchange (JEOL 7000 Specimen Exchange
How to use the Jeol 1010 TEM of GI (Liesbeth own GI version)
 How to use the Jeol 1010 TEM of GI (Liesbeth own GI version) 1.Load the specimen Load a grid into the rod holder: USE ONLY THE TOP POSITION (blue arrow), Specimen selection on 1 (The rear one is only a
How to use the Jeol 1010 TEM of GI (Liesbeth own GI version) 1.Load the specimen Load a grid into the rod holder: USE ONLY THE TOP POSITION (blue arrow), Specimen selection on 1 (The rear one is only a
MSE 460 TEM Lab 4: Bright/Dark Field Imaging Operation
 MSE 460 TEM Lab 4: Bright/Dark Field Imaging Operation Last updated on 1/8/2018 Jinsong Wu, jinsong-wu@northwestern.edu Aims: The aim of this lab is to familiarize you with bright/dark field imaging operation.
MSE 460 TEM Lab 4: Bright/Dark Field Imaging Operation Last updated on 1/8/2018 Jinsong Wu, jinsong-wu@northwestern.edu Aims: The aim of this lab is to familiarize you with bright/dark field imaging operation.
FEI Helios NanoLab 600 TEM specimen prep recipe Nicholas G. Rudawski (352) (office) (805) (cell) Last updated: 01/19/17
 FEI Helios NanoLab 600 TEM specimen prep recipe Nicholas G. Rudawski ngr@ufl.edu (352) 392 3077 (office) (805) 252-4916 (cell) Last updated: 01/19/17 This recipe is based on the methods of Schaffer et
FEI Helios NanoLab 600 TEM specimen prep recipe Nicholas G. Rudawski ngr@ufl.edu (352) 392 3077 (office) (805) 252-4916 (cell) Last updated: 01/19/17 This recipe is based on the methods of Schaffer et
Talos on-line help User interface 1 Software version 1.6 and higher
 Talos on-line help User interface 1 Talos on-line help manual -- User interface Table of Contents 1 User Interface... 3 1.1 View modes... 4 1.2 Workset tabs... 4 1.3 Control panels... 5 1.4 Popup panels...
Talos on-line help User interface 1 Talos on-line help manual -- User interface Table of Contents 1 User Interface... 3 1.1 View modes... 4 1.2 Workset tabs... 4 1.3 Control panels... 5 1.4 Popup panels...
Title: Amray 1830 SEM#2 Semiconductor & Microsystems Fabrication Laboratory Revision: D Rev Date: 03/18/2016
 Approved by: Process Engineer / / / / Equipment Engineer 1 SCOPE The purpose of this document is to detail the use of the Amray 1830 SEM. All users are expected to have read and understood this document.
Approved by: Process Engineer / / / / Equipment Engineer 1 SCOPE The purpose of this document is to detail the use of the Amray 1830 SEM. All users are expected to have read and understood this document.
KEYENCE VKX LASER-SCANNING CONFOCAL MICROSCOPE Standard Operating Procedures (updated Oct 2017)
 KEYENCE VKX LASER-SCANNING CONFOCAL MICROSCOPE Standard Operating Procedures (updated Oct 2017) 1 Introduction You must be trained to operate the Laser-scanning confocal microscope (LSCM) independently.
KEYENCE VKX LASER-SCANNING CONFOCAL MICROSCOPE Standard Operating Procedures (updated Oct 2017) 1 Introduction You must be trained to operate the Laser-scanning confocal microscope (LSCM) independently.
ScanArray Overview. Principle of Operation. Instrument Components
 ScanArray Overview The GSI Lumonics ScanArrayÒ Microarray Analysis System is a scanning laser confocal fluorescence microscope that is used to determine the fluorescence intensity of a two-dimensional
ScanArray Overview The GSI Lumonics ScanArrayÒ Microarray Analysis System is a scanning laser confocal fluorescence microscope that is used to determine the fluorescence intensity of a two-dimensional
Model SU3500 Scanning Electron Microscope
 Model SU3500 Scanning Electron Microscope Modified and Parts taken from Hitachi Easy Operation Guide. Before using the Model SU3500 SEM, be sure to read the [GENERAL SAFETY GUIDELINES] in the instruction
Model SU3500 Scanning Electron Microscope Modified and Parts taken from Hitachi Easy Operation Guide. Before using the Model SU3500 SEM, be sure to read the [GENERAL SAFETY GUIDELINES] in the instruction
Introduction of New Products
 Field Emission Electron Microscope JEM-3100F For evaluation of materials in the fields of nanoscience and nanomaterials science, TEM is required to provide resolution and analytical capabilities that can
Field Emission Electron Microscope JEM-3100F For evaluation of materials in the fields of nanoscience and nanomaterials science, TEM is required to provide resolution and analytical capabilities that can
Powder diffractometer operation instructions D8 Advance with a Cu Kα sealed tube and Lynxeye MARCH 30, 2016
 Powder diffractometer operation instructions D8 Advance with a Cu Kα sealed tube and Lynxeye MARCH 30, 2016 1 SAFETY. For radiation safety materials refer to http://www.ehs.wisc.edu/raddevices.htm. There
Powder diffractometer operation instructions D8 Advance with a Cu Kα sealed tube and Lynxeye MARCH 30, 2016 1 SAFETY. For radiation safety materials refer to http://www.ehs.wisc.edu/raddevices.htm. There
Tecnai on-line help User interface 1 Tecnai F20 Tecnai F30 User interface Software version 2.1.8/3.0
 Tecnai on-line help User interface 1 Tecnai on-line help manual -- User interface Table of Contents 1 User Interface...5 1.1 View modes...6 1.2 Toolbar...6 1.3 Workset tabs...7 1.4 Control panels...7 1.5
Tecnai on-line help User interface 1 Tecnai on-line help manual -- User interface Table of Contents 1 User Interface...5 1.1 View modes...6 1.2 Toolbar...6 1.3 Workset tabs...7 1.4 Control panels...7 1.5
SEM OPERATION IN LOW VACUUM MODE
 SEM OPERATION IN LOW VACUUM MODE Instructions for JEOL 5800 LV The EVAC light of the SEM specimen chamber should be already lit when you approach the SEM & the SEM will have been left in the high vacuum
SEM OPERATION IN LOW VACUUM MODE Instructions for JEOL 5800 LV The EVAC light of the SEM specimen chamber should be already lit when you approach the SEM & the SEM will have been left in the high vacuum
COMPACT MANUAL FOR GI USERS OF THE JEM 1400 FLASH BEGINNERS (For internal use only) Gray means additional information at the end of this mini-manual
 1 COMPACT MANUAL FOR GI USERS OF THE JEM 1400 FLASH BEGINNERS (For internal use only) Gray means additional information at the end of this mini-manual ABOUT THIS MICROSCOPE (room HG01.240) The JEM-1400Flash
1 COMPACT MANUAL FOR GI USERS OF THE JEM 1400 FLASH BEGINNERS (For internal use only) Gray means additional information at the end of this mini-manual ABOUT THIS MICROSCOPE (room HG01.240) The JEM-1400Flash
Optika ISview. Image acquisition and processing software. Instruction Manual
 Optika ISview Image acquisition and processing software Instruction Manual Key to the Instruction Manual IS is shortened name used for OptikaISview Square brackets are used to indicate items such as menu
Optika ISview Image acquisition and processing software Instruction Manual Key to the Instruction Manual IS is shortened name used for OptikaISview Square brackets are used to indicate items such as menu
Horiba Jobin-Yvon LabRam Raman Confocal Microscope (GERB 120)
 Horiba Jobin-Yvon LabRam Raman Confocal Microscope (GERB 120) Please contact Dr. Amanda Henkes for training requests and assistance: 979-862-5959, amandahenkes@tamu.edu Hardware LN 2 FTIR FTIR camera 1
Horiba Jobin-Yvon LabRam Raman Confocal Microscope (GERB 120) Please contact Dr. Amanda Henkes for training requests and assistance: 979-862-5959, amandahenkes@tamu.edu Hardware LN 2 FTIR FTIR camera 1
INSTRUCTIONS JEM-2010F FIELD-EMISSION TRANSMISSION ELECTRON MICROSCOPE WITH STEM CAPABILITY
 INSTRUCTIONS JEM-2010F FIELD-EMISSION TRANSMISSION ELECTRON MICROSCOPE WITH STEM CAPABILITY August 2011 PRELIMINARIES OPERATION 1. Ensure that EMISSION and HT are on: The HT READY and FEG READY lights
INSTRUCTIONS JEM-2010F FIELD-EMISSION TRANSMISSION ELECTRON MICROSCOPE WITH STEM CAPABILITY August 2011 PRELIMINARIES OPERATION 1. Ensure that EMISSION and HT are on: The HT READY and FEG READY lights
BX-61: Brightfield Instruction /Continue to scroll for Fluorescent Instuctions
 BX-61: Brightfield Instruction /Continue to scroll for Fluorescent Instuctions Starting up: Schematic of Olympus BX-61. 1. Turn on Olympus microscope power box (left of microscope) with toggle switch on
BX-61: Brightfield Instruction /Continue to scroll for Fluorescent Instuctions Starting up: Schematic of Olympus BX-61. 1. Turn on Olympus microscope power box (left of microscope) with toggle switch on
Cryogenic Transmission Electron Microscope
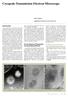 Cryogenic Transmission Electron Microscope Hideo Nishioka Application & Research Center, JEOL Ltd. Introduction The transmission electron microscope (TEM) that has been widely used in research in the fields
Cryogenic Transmission Electron Microscope Hideo Nishioka Application & Research Center, JEOL Ltd. Introduction The transmission electron microscope (TEM) that has been widely used in research in the fields
JEOL JEM 2010 TRAINING TRANSMISSION ELECTRON MICROSCOPE USER MANUAL
 JEOL JEM 2010 TRAINING TRANSMISSION ELECTRON MICROSCOPE USER MANUAL Version 5.1 EM Facility CMSE-SEF Massachusetts Institution of Technology TABLE OF CONTENTS 1. Specifications...2 1.1 Performance...2
JEOL JEM 2010 TRAINING TRANSMISSION ELECTRON MICROSCOPE USER MANUAL Version 5.1 EM Facility CMSE-SEF Massachusetts Institution of Technology TABLE OF CONTENTS 1. Specifications...2 1.1 Performance...2
Therefore, all descriptions and illustrations in this instruction manual, including all specifications are subject to change without notice.
 We are constantly endeavouring to improve our instruments and to adapt them to the requirements of modern research techniques and testing methods. This involves modification to the mechanical structure
We are constantly endeavouring to improve our instruments and to adapt them to the requirements of modern research techniques and testing methods. This involves modification to the mechanical structure
Dickinson College Department of Geology
 Dickinson College Department of Geology Title: Equipment: BASIC OPERATION OF THE SCANNING ELECTRON MICROSCOPE (SEM) JEOL JSM-5900 SCANNING ELECTRON MICROSCOPE Revision: 2.2 Effective Date: 1/29/2003 Author(s):
Dickinson College Department of Geology Title: Equipment: BASIC OPERATION OF THE SCANNING ELECTRON MICROSCOPE (SEM) JEOL JSM-5900 SCANNING ELECTRON MICROSCOPE Revision: 2.2 Effective Date: 1/29/2003 Author(s):
STEM Spectrum Imaging Tutorial
 STEM Spectrum Imaging Tutorial Gatan, Inc. 5933 Coronado Lane, Pleasanton, CA 94588 Tel: (925) 463-0200 Fax: (925) 463-0204 April 2001 Contents 1 Introduction 1.1 What is Spectrum Imaging? 2 Hardware 3
STEM Spectrum Imaging Tutorial Gatan, Inc. 5933 Coronado Lane, Pleasanton, CA 94588 Tel: (925) 463-0200 Fax: (925) 463-0204 April 2001 Contents 1 Introduction 1.1 What is Spectrum Imaging? 2 Hardware 3
AgilEye Manual Version 2.0 February 28, 2007
 AgilEye Manual Version 2.0 February 28, 2007 1717 Louisiana NE Suite 202 Albuquerque, NM 87110 (505) 268-4742 support@agiloptics.com 2 (505) 268-4742 v. 2.0 February 07, 2007 3 Introduction AgilEye Wavefront
AgilEye Manual Version 2.0 February 28, 2007 1717 Louisiana NE Suite 202 Albuquerque, NM 87110 (505) 268-4742 support@agiloptics.com 2 (505) 268-4742 v. 2.0 February 07, 2007 3 Introduction AgilEye Wavefront
Figure 1 The Raith 150 TWO
 RAITH 150 TWO SOP Figure 1 The Raith 150 TWO LOCATION: Raith 150 TWO room, Lithography area, NanoFab PRIMARY TRAINER: SECONDARY TRAINER: 1. OVERVIEW The Raith 150 TWO is an ultra high resolution, low voltage
RAITH 150 TWO SOP Figure 1 The Raith 150 TWO LOCATION: Raith 150 TWO room, Lithography area, NanoFab PRIMARY TRAINER: SECONDARY TRAINER: 1. OVERVIEW The Raith 150 TWO is an ultra high resolution, low voltage
Leica DMi8A Quick Guide
 Leica DMi8A Quick Guide 1 Optical Microscope Quick Start Guide The following instructions are provided as a Quick Start Guide for powering up, running measurements, and shutting down Leica s DMi8A Inverted
Leica DMi8A Quick Guide 1 Optical Microscope Quick Start Guide The following instructions are provided as a Quick Start Guide for powering up, running measurements, and shutting down Leica s DMi8A Inverted
AxioVision 4.5 Brightfield Image Capture Procedure
 AxioVision 4.5 Brightfield Image Capture Procedure 1. STARTING-UP PROCEDURE: Remove blue dust cover and place on shelf under microscope. Turn on the halogen lamp by pushing the switch at the back right
AxioVision 4.5 Brightfield Image Capture Procedure 1. STARTING-UP PROCEDURE: Remove blue dust cover and place on shelf under microscope. Turn on the halogen lamp by pushing the switch at the back right
Tecnai on-line help manual --
 Tecnai on-line help Alignments 1 Tecnai on-line help manual -- Alignments Table of Contents 1 Alignments in the Tecnai microscope...5 2 Alignment procedures...6 3 Introduction to electron optics...11 3.1
Tecnai on-line help Alignments 1 Tecnai on-line help manual -- Alignments Table of Contents 1 Alignments in the Tecnai microscope...5 2 Alignment procedures...6 3 Introduction to electron optics...11 3.1
FE-SEM SU-8020 Operating manual (Preliminary version)
 FE-SEM SU-8020 Operating manual (Preliminary version) 2016/04/11 Seimitsu Bunseki sitsu lab. Starting up 1.Turn on the Display switch. Windows OS is starting up 2. Select the user SU-8000. 3. Click the
FE-SEM SU-8020 Operating manual (Preliminary version) 2016/04/11 Seimitsu Bunseki sitsu lab. Starting up 1.Turn on the Display switch. Windows OS is starting up 2. Select the user SU-8000. 3. Click the
General information. If you see the instrument turned off, notify MIC personnel. MIC personnel will help you insert your samples into the instrument.
 JEOL JSM-7400F Table of contents General information.. 3 The operation panel. 4 The different sample holders and inserting the samples.. 5 Turning on the beam... 6 Stage map control... 8 Correcting astigmatism...
JEOL JSM-7400F Table of contents General information.. 3 The operation panel. 4 The different sample holders and inserting the samples.. 5 Turning on the beam... 6 Stage map control... 8 Correcting astigmatism...
SerialEM Help Index. ABC Amber HLP Converte r T rial version,
 SerialEM Help Index Genera l Topics Introduction to SerialEM Mouse and Keyboard Controls Control Panels Image Acquisition Acquiring Tilt Series Montaging Low Dose Mode Energy Filtering Using the Navigator
SerialEM Help Index Genera l Topics Introduction to SerialEM Mouse and Keyboard Controls Control Panels Image Acquisition Acquiring Tilt Series Montaging Low Dose Mode Energy Filtering Using the Navigator
CALIBRATION OF MICROSCOPE EYEPIECE GRATICULE
 CALIBRATION OF MICROSCOPE EYEPIECE GRATICULE A typical eyepiece graticule looks like this: It is 10mm in length and each mm is divided into 10 parts So each small division = 0.1mm = 100µm The eyepiece
CALIBRATION OF MICROSCOPE EYEPIECE GRATICULE A typical eyepiece graticule looks like this: It is 10mm in length and each mm is divided into 10 parts So each small division = 0.1mm = 100µm The eyepiece
SCIENTIFIC INSTRUMENT NEWS. Introduction. Design of the FlexSEM 1000
 SCIENTIFIC INSTRUMENT NEWS 2017 Vol. 9 SEPTEMBER Technical magazine of Electron Microscope and Analytical Instruments. Technical Explanation The FlexSEM 1000: A Scanning Electron Microscope Specializing
SCIENTIFIC INSTRUMENT NEWS 2017 Vol. 9 SEPTEMBER Technical magazine of Electron Microscope and Analytical Instruments. Technical Explanation The FlexSEM 1000: A Scanning Electron Microscope Specializing
Photoshop Elements Hints by Steve Miller
 2015 Elements 13 A brief tutorial for basic photo file processing To begin, click on the Elements 13 icon, click on Photo Editor in the first box that appears. We will not be discussing the Organizer portion
2015 Elements 13 A brief tutorial for basic photo file processing To begin, click on the Elements 13 icon, click on Photo Editor in the first box that appears. We will not be discussing the Organizer portion
The ideal K-12 science microscope solution. User Guide. for use with the Nova5000
 The ideal K-12 science microscope solution User Guide for use with the Nova5000 NovaScope User Guide Information in this document is subject to change without notice. 2009 Fourier Systems Ltd. All rights
The ideal K-12 science microscope solution User Guide for use with the Nova5000 NovaScope User Guide Information in this document is subject to change without notice. 2009 Fourier Systems Ltd. All rights
Strata DB235 FESEM FIB
 Strata DB235 FESEM FIB Standard Operating Procedure Revision: 5.0 Last Updated: August 16/2016, revised by Li Yang Overview This document will provide a detailed operation procedure of the Focused Ion
Strata DB235 FESEM FIB Standard Operating Procedure Revision: 5.0 Last Updated: August 16/2016, revised by Li Yang Overview This document will provide a detailed operation procedure of the Focused Ion
Operation Guide for the Leica SP2 Confocal Microscope Bio-Imaging Facility Hunter College October 2009
 Operation Guide for the Leica SP2 Confocal Microscope Bio-Imaging Facility Hunter College October 2009 Introduction of Fluoresence Confocal Microscopy The first confocal microscope was invented by Princeton
Operation Guide for the Leica SP2 Confocal Microscope Bio-Imaging Facility Hunter College October 2009 Introduction of Fluoresence Confocal Microscopy The first confocal microscope was invented by Princeton
SEM Training Notebook
 SEM Training Notebook Lab Manager: Dr. Perry Cheung MSE Fee-For-Service Facility Materials Science and Engineering University of California, Riverside December 21, 2017 (rev. 3.4) 1 Before you begin Complete
SEM Training Notebook Lab Manager: Dr. Perry Cheung MSE Fee-For-Service Facility Materials Science and Engineering University of California, Riverside December 21, 2017 (rev. 3.4) 1 Before you begin Complete
Standard Operating Procedure of Atomic Force Microscope (Anasys afm+)
 Standard Operating Procedure of Atomic Force Microscope (Anasys afm+) The Anasys Instruments afm+ system incorporates an Atomic Force Microscope which can scan the sample in the contact mode and generate
Standard Operating Procedure of Atomic Force Microscope (Anasys afm+) The Anasys Instruments afm+ system incorporates an Atomic Force Microscope which can scan the sample in the contact mode and generate
ML7520 ML7530 DIOPTER ADJUSTMENT RING BINOCULAR BODY, INCLINED 30. (a) Field Iris Control Lever. (c) Filter Slots EYEPIECES, KHW10X
 JAPAN DIOPTER ADJUSTMENT RING BINOCULAR BODY, INCLINED 30 (a) Field Iris Control Lever (c) Filter Slots EYEPIECES, KHW10X ANALYZER CONTROL LEVER (b) Aperture Iris Control Lever LIGHT SOURCE HOUSING VERTICAL
JAPAN DIOPTER ADJUSTMENT RING BINOCULAR BODY, INCLINED 30 (a) Field Iris Control Lever (c) Filter Slots EYEPIECES, KHW10X ANALYZER CONTROL LEVER (b) Aperture Iris Control Lever LIGHT SOURCE HOUSING VERTICAL
CTF Correction with IMOD
 CTF Correction with IMOD CTF Correction When microscope is operated in underfocus to produce phase contrast, the contrast is inverted in some spatial frequency ranges 1 We See Only Amplitudes, Not Phases,
CTF Correction with IMOD CTF Correction When microscope is operated in underfocus to produce phase contrast, the contrast is inverted in some spatial frequency ranges 1 We See Only Amplitudes, Not Phases,
SEM Training Notebook
 SEM Training Notebook Lab Manager: Dr. Perry Cheung MSE Fee-For-Service Facility Materials Science and Engineering University of California, Riverside March 8, 2018 (rev. 3.5) 1 Before you begin Complete
SEM Training Notebook Lab Manager: Dr. Perry Cheung MSE Fee-For-Service Facility Materials Science and Engineering University of California, Riverside March 8, 2018 (rev. 3.5) 1 Before you begin Complete
Digital Portable Overhead Document Camera LV-1010
 Digital Portable Overhead Document Camera LV-1010 Instruction Manual 1 Content I Product Introduction 1.1 Product appearance..3 1.2 Main functions and features of the product.3 1.3 Production specifications.4
Digital Portable Overhead Document Camera LV-1010 Instruction Manual 1 Content I Product Introduction 1.1 Product appearance..3 1.2 Main functions and features of the product.3 1.3 Production specifications.4
