SUPPLEMENTARY METHODS. PIN2-Dendra2 transgenic line creation
|
|
|
- Bathsheba Barrett
- 6 years ago
- Views:
Transcription
1 SUPPLEMENTARY METHODS PIN2-Dendra2 transgenic line creation The PIN2-Dendra2 construct was described previously (Jásik et al., 2013). Shortly, for generation of the expressing vector encompassing the PIN2-Dendra2 fusion, DNA fragments of PIN2 including upstream and downstream regulatory sequences were multiplied by PCR from genomic DNA of Arabidopsis thaliana ecotype Columbia (Col-0), Dendra2 was amplified by PCR from a Gateway Dendra2-At-N entry clone (Evrogen, Moscow, Russia). Fragments were cloned sequentially into the pampat-msc vector (Genank: AY ) such that Dendra2 was positioned in the PIN2 intracytoplasmic loop downstream of Alanine 403. The construct was transformed into the Arabidopsis thaliana Col-0 accession using Agrobacterium tumefaciens GV3101(pMP90RK) by the floral-dip method. Selection of homozygous plants was performed by growing seedlings on medium supplemented with 7.5 mg/l phosphinothricin (PPT, Duchefa, Haarlem, The Netherlands). Plant material, cultivation conditions, media and chemicals Seeds were surface sterilized with 1% (w/v) sodium hypochlorite and germinated in Petri dishes with MSMO medium, diluted 1:1 (Sigma, #M6899) supplemented with 1% sucrose and solidified by 0.8% agar. Medium ph was adjusted to 5.8 before autoclaving. Dishes were kept in vertical position in a growth chamber at 21 ºC under continuous light (100 μmol m -2 s -1 ). efore performing the experiments, seedlings were transferred onto fresh medium for 12 h. Treatments of seedlings was performed on microscope slides covered with 1.5 ml solid culture medium containing drugs of interest. Four to five seedlings were used per slide and experiments were repeated three times. Slides were placed on the culture medium in Petri dishes and stored vertical under light and at 21 C. Antibiotics and refeldin A (FA) were dissolved in ethanol, Tyrphostin A 23 in DMSO. Concentration of ethanol in the medium did not exceed 0.1%, DMSO 0.05% (v/v). Cycloheximidine (CHX) and FA were used in concentration of 50 μm (if not indicated differently), Tyrphostin A23
2 in concentration of 100 μm, actinomycin A in concentration of 50 mg/l. Control treatments were performed with equal amounts of solvent. Medium ph was rechecked after drug supplementation and adjusted if required to the same value as control medium. Tyrphostin A23 was from Calbiochem (San Diego, USA), other chemicals were from Sigma-Aldrich (St. Louis, USA). Photoconversion and microscopy efore photoconversion and imaging, seedlings were covered by a coverslip which was removed gently again after imaging. The general scheme for time course analysis was as follows: roots were imaged in both channels (green and red fluorescence), photoconverted, again imaged and then reimaged at different time points during the experiment under the same microscope settings. Photoconversion of roots was performed for 15 s with a 100W mercury lamp, a 20 x /0.75 Plan Apochromat objective and an excitation filter P 405/5 nm of the inverted Zeiss Axiovert 200M microscope of the confocal laser scanning system (LSM 510 Meta, Carl Zeiss Jena, Germany) For quantitative analysis of PIN 2 abundance images were acquired with 8-bit color depth using a Zeiss LSM-510 META with the 20 x /0.75 Plan Apochromat objective and pinhole setting at 140 μm (4 μm optical slice). Red and green signals were acquired in a multi-track mode. For green signal capture, samples were excited with the 488-nm line of the argon laser and the signal was collected with a 505 to 530 nm band-pass filter. For red signal, samples were excited by the 543-nm line of the HeNe laser and fluorescence was collected with a 565 to 615 nm band-pass filter. For both channels the HFT UV/488/543/633 was used as main dichroic mirror. Gain and offset parameters of the LSM detector and laser line power in the photoconverted experiments were set such way that the green signal of the unconverted PM and the red signal of the photoconverted PM gave approximately the same values of around 70 arbitrary units of the 0 to 256 scale of the 8-bit color depth mode. Identical settings were kept to collect images at all time points within experiments. For emission spectra analysis, different excitation laser lines in combination with appropriate main dichroic mirrors were applied and fluorescence emissions were collected with the spectral Meta detector (lambda-mode).
3 Data evaluation and presentation Mean fluorescence intensities of the transversal PM in root epidermis were quantified by ImageJ software (NIH, ethesda, USA) using the straight line selection mode. Fifteen PMs were investigated at every time point in every root of four to five seedlings used per slide. Results in graphs are presented as normalized data if not otherwise stated. In Figure 1A, D and F at every time point the means of green signal intensities of PM in individual roots were normalized to the mean of green signal intensities emitted by PM of the same root before conversion. Time point 0 represents the relative green signal intensity recorded immediately after conversion. The means of red signal intensities of PM in individual roots at every time point were normalized to the mean red fluorescence intensity measured soon after conversion (time point 0). Values of the time points 0 were set to 1. The means of green signal intensities of FA-Cs in Figure 1A at every time point were normalized to the mean of green signal intensities emitted by PM of the same root before conversion. In a similar way the red signal intensities of FA-Cs at every time point were related to the mean red fluorescence intensity of PM measured immediately after conversion. In Figure 1C (right panel) in single roots the means of green signal intensities of FA-Cs at every time point during the experiment were normalized to the mean of green signal intensities emitted by FA-Cs of the same root before photoconversion. Time point 0 represents relative green signal intensity recorded immediately after photoconversion. The means of red signal intensities of FA-Cs were related to the mean red fluorescence intensity of FA-Cs measured immediately after photoconversion (time point 0). The value of the time points 0 was set to 1. In Figure 1G samples were imaged in both channels after 2.5 h treatment with FA and washing (time point 0). In single roots the means of green and red signal intensities of PM at every time point during the experiment were normalized to the means of green and red signal intensities emitted by the PM at time point 0. Values for green and red signal intensities of the time points 0 were set to 1. Graphs were prepared in Microsoft Excel (Microsoft, Redmont, USA). Points displayed at graphs represent means of normalized values of 12 to15 roots; bars correspond to standard errors of the means (SE). Images were processed with Adobe Photoshop CS2 (Adobe Systems, Mountain View, USA) and Microsoft Publisher (Microsoft, Redmont, USA) softwares.
4 SUPLEMENTARY FIGURES Supplementary Figure 1. Time course pattern of PIN2 populations in PM and FA-Cs. Images taken in green and red channels show decreasing red signal intensity and increasing green signal intensity in PM during the experiment. Note the prevalence of the green signal in FA-Cs at every time point from 2 to 12 h. Roots were imaged before and after photoconversion, then moved onto medium supplied with 50 μm FA and reimaged several times within 12 h under the same microscope settings. Scale bar = 20 μm.
5 Supplementary Figure 2. Comparison of spectra emitted by PM and FA-Cs. Spectra were measured with a Zeiss LSM- 510 microscope using the meta detector and the lambda acquisition mode in roots treated with 50 μm FA for 2 h. Roots were either photoconverted (marked as converted ) or not photoconverted (marked as unconverted ) and excited by the different laser lines. A region of interest 1 (ROI1) drawn in the red color in coded images represents FA-Cs; a region of interest 2 (ROI2) drawn in green color corresponds to PM. Red lines in related graphs show the emission spectrum patterns obtained by analysis of ROI1, the green lines represent ROI2.
6 C D Supplementary Figure 3. Monitoring of FA-Cs development with unconverted PIN2-Dendra2. Note that FA-Cs in seedlings grown on medium with 50 μm FA were initially small and reached full size within 2 h (A). The number of FA body s per cell slightly decreased during the extended cultivation of seedlings on FA () while FA-Cs size (C) and fluorescence signal intensity emitted by FA-Cs (D) increased dramatically within the first 2 h. Later on these parameters were more or less constant. In D at every time point the means of signal intensities of FA-Cs in individual roots were normalized to the mean of green signal intensities emitted by PM of the same root before conversion. In A the scale bar = 5 μm
7 time (h) Supplementary Figure 4. Effect of CHX and actinomycin A on PIN2 abundance in FA-Cs and PM. After pretreatment of seedlings by antibiotics and photoconversion, FA-Cs still contained the red PIN2-Dendra2 form but not the green PIN-Dendra2 variety (A). Quantitative data for actinomycin A and CHX effects are given in. Samples marked as converted were pretreated either with 50 mg/l actinomycin A or 50 μm CHX for 1.5 h, then placed on medium with the appropriate antibiotic and 50 μm FA, imaged, photoconverted, again imaged and then reimaged at different time points. In the unconverted experiment samples were also pretreated by antibiotics but not photoconverted. FA-Cs means of red signal intensities in the converted experiment at every time point during the experiment were normalized to the mean of red signal intensities emitted by PM of the same root immediately after conversion. In an unconverted approach FA-Cs means of green signal intensities were related to the mean of green signal intensities emitted by PM of the same root after 1.5 h antibiotic pretreatment. Scale bar = 10 μm.
8 C D Supplementary Figure 5. Disappearance of FA-Cs from cells after FA washingout. The number of cells with FA-Cs (A), number of FA-Cs per cell (), size of FA-Cs (C) and red signal intensity in FA-Cs (D) decreased rapidly after FA was washed out. Seedlings were kept on medium supplemented with 50 μm FA for 2.5 h, then rinsed for 10 s with liquid culture medium, placed on FA-free medium, photoconverted, imaged in the red channel (time point 0) and then reimaged at different time points. Control seedlings were not washed and were kept permanently on medium with FA. Their roots were also converted after 2.5 h, imaged (time point 0) and reimaged at the same time points as rinsed roots. In C the means of FA- Cs sizes in individual roots at each time point were normalized to the mean size of FA-Cs of the same root measured immediately after conversion (time point 0). The values of the time points 0 were set to 1. In D the means of red signal intensities emitted by FA-Cs in individual roots at every time point were related to the mean of red fluorescence intensity of FA-Cs measured in the same roots immediately after conversion (time point 0). The values of the time points 0 were set to 1.
9 Supplementary Figure 6. PIN2 abundance in PM after FA washing-out. When seedlings pretreated with 50 μm FA were rinsed and placed on FA-free medium, FA-Cs disappeared and the intensity of fluorescence signal in PM increased (A, marked as washed ), on the other hand samples permanently growing on FA medium showed no obvious differences (marked as not washed ). Graph in shows data obtained by the measurement of membrane intensities at different time points. PIN2-Dendra2 seedlings were grown on medium with 50 μm FA for 2.5 h and then after 10 s washing with liquid culture medium were placed on medium without FA, imaged in green channel (time point 0) and reimaged at different time points. Control seedlings grown permanently either with or without FA were imaged the first time after 2.5 h (time point 0) of cultivation and reimaged as shown in the graph. In single roots the means of green signal intensities at every time point during the experiment were normalized to the mean of green signal intensities emitted by PM of the same root at time point 0. Values of the time points 0 were set to 1. Scale bars = 10 μm
10 Supplementary Figure 7. The abundance of PIN2 in PM in the presence of CHX after FA washing-out. FA-Cs developed after photoconversion in the presence of CHX contained red signal and disappeared from the cells after washing. However, this vanishing did not increase the red signal intensity in PM (A). Quantitative data are given in. Seedlings were pretreated with 50 μm CHX for 1 h, photoconverted, treated with 50 μm FA in the presence of CHX for 2.5 h and then after 10 s washing with liquid culture medium they were placed on medium with CHX but without FA, imaged in red channel (time point 0) and reimaged at different time points. In single roots the means of red signal intensities at every time point during the experiment were normalized to the mean of red signal intensities emitted by the PM of the same root at time point 0. Values of the time points 0 were set to 1. Scale bars = 10 μm
11 Supplementary Figure 8. Effect of tyrphostin A23 (A23) on PIN2 turnover in PM and FA-Cs. A23 inhibits recovery of PIN2-Dendra2 green fluorescent pool in PM and inhibits PIN2 internalization (A) and affects the proportion of secreted and internalized PIN2-Dendra2 in FA-Cs (). Seedlings were treated with100 μm A23 for 30 min, then placed on medium either with (A23 pretreated + FA) or without 50 μm FA (A23 pretreated), imaged, photoconverted and reimaged. The control was kept on medium without A23 and FA; samples A23 continuously were cultivated permanently on medium with 100 μm A23. In both cases roots were imaged, after 30 min of cultivation, photoconverted and reimaged. In single roots the means of green fluorescence signal intensities of PM at every time point during the experiment were normalized to the mean of green signal intensities emitted by PM of the same root before conversion. Time points 0 represent relative signal intensities recorded after conversion. Red signal intensities were related to the mean red signal intensities measured immediately after conversion (time point 0). Values of the time points 0 were set to 1.In samples were treated with A23 for 30 min, than placed on medium supplemented with 50 μm FA, imaged, photoconverted and again imaged. Control samples were not pretreated with A23. In single roots, the means of green signal intensities of FA- Cs at every time point during the experiment were related to the mean of green signal intensities emitted by PM of the same root before photoconversion. Red signal intensities at every time point were related to the mean red fluorescence intensity of PM measured immediatelly after conversion.
ZEISS LSM510META confocal manual
 ZEISS LSM510META confocal manual Switching on the system 1) Switch on the Remote Control button located on the table to the right of the microscope. This is the main switch for the whole system including
ZEISS LSM510META confocal manual Switching on the system 1) Switch on the Remote Control button located on the table to the right of the microscope. This is the main switch for the whole system including
Practical work no. 3: Confocal Live Cell Microscopy
 Practical work no. 3: Confocal Live Cell Microscopy Course Instructor: Mikko Liljeström (MIU) 1 Background Confocal microscopy: The main idea behind confocality is that it suppresses the signal outside
Practical work no. 3: Confocal Live Cell Microscopy Course Instructor: Mikko Liljeström (MIU) 1 Background Confocal microscopy: The main idea behind confocality is that it suppresses the signal outside
LSM 510 Meta Training Notes
 LSM 510 Meta Training Notes Turning on the system Turn on X-Cite power supply. This supplies light for epifluorescence for viewing your samples through the microscope. Turn on the remote control switch.
LSM 510 Meta Training Notes Turning on the system Turn on X-Cite power supply. This supplies light for epifluorescence for viewing your samples through the microscope. Turn on the remote control switch.
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1126551/dc1 Supporting Online Material for Visualization of Cellulose Synthase Demonstrates Functional Association with Microtubules Alex R. Paredez, Christopher R.
www.sciencemag.org/cgi/content/full/1126551/dc1 Supporting Online Material for Visualization of Cellulose Synthase Demonstrates Functional Association with Microtubules Alex R. Paredez, Christopher R.
Zeiss LSM 510 Confocor III Training Notes. Center for Cell Analysis & Modeling
 Zeiss LSM 510 Confocor III Training Notes Center for Cell Analysis & Modeling Confocor 3 Start Up Go to System Module Turn on Main Switch, System/ PC, and Components Switches Do you need the arc lamp?
Zeiss LSM 510 Confocor III Training Notes Center for Cell Analysis & Modeling Confocor 3 Start Up Go to System Module Turn on Main Switch, System/ PC, and Components Switches Do you need the arc lamp?
LSM 710 Confocal Microscope Standard Operation Protocol
 LSM 710 Confocal Microscope Standard Operation Protocol Basic Operation Turning on the system 1. Switch on Main power switch 2. Switch on System / PC power button 3. Switch on Components power button 4.
LSM 710 Confocal Microscope Standard Operation Protocol Basic Operation Turning on the system 1. Switch on Main power switch 2. Switch on System / PC power button 3. Switch on Components power button 4.
LSM 780 Confocal Microscope Standard Operation Protocol
 LSM 780 Confocal Microscope Standard Operation Protocol Basic Operation Turning on the system 1. Sign on log sheet according to Actual start time 2. Check Compressed Air supply for the air table 3. Switch
LSM 780 Confocal Microscope Standard Operation Protocol Basic Operation Turning on the system 1. Sign on log sheet according to Actual start time 2. Check Compressed Air supply for the air table 3. Switch
LSM 510 Training Notes
 LSM 510 Training Notes Turning on the system Turn on the arc lamp, found on the bench top left of the microscope. This supplies light for epifluorescence for viewing your samples through the microscope.
LSM 510 Training Notes Turning on the system Turn on the arc lamp, found on the bench top left of the microscope. This supplies light for epifluorescence for viewing your samples through the microscope.
Internal Medicine Imaging Core Emory University Department of Medicine
 Internal Medicine Imaging Core Emory University Department of Medicine 1 OPERATION OF THE ZEISS LSM 510 META YOU MUST SIGN UP TO USE THE MICROSCOPE OR COMPUTER EVERY TIME NO EXCEPTIONS Before attempting
Internal Medicine Imaging Core Emory University Department of Medicine 1 OPERATION OF THE ZEISS LSM 510 META YOU MUST SIGN UP TO USE THE MICROSCOPE OR COMPUTER EVERY TIME NO EXCEPTIONS Before attempting
Multifluorescence The Crosstalk Problem and Its Solution
 Multifluorescence The Crosstalk Problem and Its Solution If a specimen is labeled with more than one fluorochrome, each image channel should only show the emission signal of one of them. If, in a specimen
Multifluorescence The Crosstalk Problem and Its Solution If a specimen is labeled with more than one fluorochrome, each image channel should only show the emission signal of one of them. If, in a specimen
Experimental protocol PIPE
 Experimental protocol PIPE May 5, 2016 Abstract PIPE is a uorescence perturbation technique that works by measuring the expansion of a laser induced perturbation of photo convertible fused protein in the
Experimental protocol PIPE May 5, 2016 Abstract PIPE is a uorescence perturbation technique that works by measuring the expansion of a laser induced perturbation of photo convertible fused protein in the
Training Guide for Carl Zeiss LSM 510 META Confocal Microscope
 Training Guide for Carl Zeiss LSM 510 META Confocal Microscope AIM 4.2 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Turn ON Components and System/PC switches
Training Guide for Carl Zeiss LSM 510 META Confocal Microscope AIM 4.2 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Turn ON Components and System/PC switches
Supplementary Information. Stochastic Optical Reconstruction Microscopy Imaging of Microtubule Arrays in Intact Arabidopsis thaliana Seedling Roots
 Supplementary Information Stochastic Optical Reconstruction Microscopy Imaging of Microtubule Arrays in Intact Arabidopsis thaliana Seedling Roots Bin Dong 1,, Xiaochen Yang 2,, Shaobin Zhu 1, Diane C.
Supplementary Information Stochastic Optical Reconstruction Microscopy Imaging of Microtubule Arrays in Intact Arabidopsis thaliana Seedling Roots Bin Dong 1,, Xiaochen Yang 2,, Shaobin Zhu 1, Diane C.
SHORT INSTRUCTIONS FOR OPERATING LSM1/2 (Zeiss LSM510) AT CIAN Version 1.4, September 2014
 CIAN LSM1 or LSM2 short instructions, version 1.4, September 2014 page 1 of 6 SHORT INSTRUCTIONS FOR OPERATING LSM1/2 (Zeiss LSM510) AT CIAN Version 1.4, September 2014 Before starting To work with LSM1
CIAN LSM1 or LSM2 short instructions, version 1.4, September 2014 page 1 of 6 SHORT INSTRUCTIONS FOR OPERATING LSM1/2 (Zeiss LSM510) AT CIAN Version 1.4, September 2014 Before starting To work with LSM1
Why and How? Daniel Gitler Dept. of Physiology Ben-Gurion University of the Negev. Microscopy course, Michmoret Dec 2005
 Why and How? Daniel Gitler Dept. of Physiology Ben-Gurion University of the Negev Why use confocal microscopy? Principles of the laser scanning confocal microscope. Image resolution. Manipulating the
Why and How? Daniel Gitler Dept. of Physiology Ben-Gurion University of the Negev Why use confocal microscopy? Principles of the laser scanning confocal microscope. Image resolution. Manipulating the
Quick Guide. LSM 5 MP, LSM 510 and LSM 510 META. Laser Scanning Microscopes. We make it visible. M i c r o s c o p y f r o m C a r l Z e i s s
 LSM 5 MP, LSM 510 and LSM 510 META M i c r o s c o p y f r o m C a r l Z e i s s Quick Guide Laser Scanning Microscopes LSM Software ZEN 2007 August 2007 We make it visible. Contents Page Contents... 1
LSM 5 MP, LSM 510 and LSM 510 META M i c r o s c o p y f r o m C a r l Z e i s s Quick Guide Laser Scanning Microscopes LSM Software ZEN 2007 August 2007 We make it visible. Contents Page Contents... 1
TRAINING MANUAL. Multiphoton Microscopy LSM 510 META-NLO
 TRAINING MANUAL Multiphoton Microscopy LSM 510 META-NLO September 2010 Multiphoton Microscopy Training Manual Multiphoton microscopy is only available on the LSM 510 META-NLO system. This system is equipped
TRAINING MANUAL Multiphoton Microscopy LSM 510 META-NLO September 2010 Multiphoton Microscopy Training Manual Multiphoton microscopy is only available on the LSM 510 META-NLO system. This system is equipped
We attempted to separate the two dyes by acquiring images using a single excitation wavelength and just two emission wavelengths.
 TN437: Spectral Separation of monochrome images using Volocity 4.0 Introduction Spectral Separation is a technique that allows the user to separate images containing data from more than one fluorochrome
TN437: Spectral Separation of monochrome images using Volocity 4.0 Introduction Spectral Separation is a technique that allows the user to separate images containing data from more than one fluorochrome
Microscopy from Carl Zeiss
 Microscopy from Carl Zeiss Contents Page Contents... 1 Introduction... 1 Starting the System... 2 Introduction to ZEN Efficient Navigation... 5 Setting up the microscope... 10 Configuring the beam path
Microscopy from Carl Zeiss Contents Page Contents... 1 Introduction... 1 Starting the System... 2 Introduction to ZEN Efficient Navigation... 5 Setting up the microscope... 10 Configuring the beam path
Things to check before start-up.
 Byeong Cha Page 1 11/24/2009 Manual for Leica SP2 Confocal Microscope Enter you name, the date, the time, and the account number in the user log book. Things to check before start-up. Make sure that your
Byeong Cha Page 1 11/24/2009 Manual for Leica SP2 Confocal Microscope Enter you name, the date, the time, and the account number in the user log book. Things to check before start-up. Make sure that your
Confocal Laser Scanning Microscopy
 Name of the Core Facility: Confocal Laser Scanning Microscopy CORE Forschungszentrum Immunologie Mainz Welcome to the CSLM Core Facility: The CLSM Core Facility enables working groups to incorporate high
Name of the Core Facility: Confocal Laser Scanning Microscopy CORE Forschungszentrum Immunologie Mainz Welcome to the CSLM Core Facility: The CLSM Core Facility enables working groups to incorporate high
Comparing FCS and FRAP as methodologies for calculating diffusion
 Bi/BE 227 Winter 2018 Assignment #4 Comparing FCS and FRAP as methodologies for calculating diffusion Schedule: Jan 29: Assignment Jan 29-Feb 14: Work on assignment Feb 14: Student PowerPoint presentations.
Bi/BE 227 Winter 2018 Assignment #4 Comparing FCS and FRAP as methodologies for calculating diffusion Schedule: Jan 29: Assignment Jan 29-Feb 14: Work on assignment Feb 14: Student PowerPoint presentations.
Supplemental Method Information Zeiss LSM710
 Supplemental Method Information Zeiss LSM710 1 Under the Light Path window set up the confocal for imaging a green dye (Alexa488-EGFP). For example, set up the light path as shown here using the 488 nm
Supplemental Method Information Zeiss LSM710 1 Under the Light Path window set up the confocal for imaging a green dye (Alexa488-EGFP). For example, set up the light path as shown here using the 488 nm
Akinori Mitani and Geoff Weiner BGGN 266 Spring 2013 Non-linear optics final report. Introduction and Background
 Akinori Mitani and Geoff Weiner BGGN 266 Spring 2013 Non-linear optics final report Introduction and Background Two-photon microscopy is a type of fluorescence microscopy using two-photon excitation. It
Akinori Mitani and Geoff Weiner BGGN 266 Spring 2013 Non-linear optics final report Introduction and Background Two-photon microscopy is a type of fluorescence microscopy using two-photon excitation. It
CONFOCAL MICROSCOPE (Zeiss LSM 510 META v4.2)
 Wellcome Trust Centre for Human Genetics Molecular Cytogenetics and Microscopy Core CONFOCAL MICROSCOPE (Zeiss LSM 510 META v4.2) 1) STARTING THE SYSTEM Abridged INSTRUCTIONS Switch on the mercury bulb
Wellcome Trust Centre for Human Genetics Molecular Cytogenetics and Microscopy Core CONFOCAL MICROSCOPE (Zeiss LSM 510 META v4.2) 1) STARTING THE SYSTEM Abridged INSTRUCTIONS Switch on the mercury bulb
Operating Instructions for Zeiss LSM 510
 Operating Instructions for Zeiss LSM 510 Location: GNL 6.312q (BSL3) Questions? Contact: Maxim Ivannikov, maivanni@utmb.edu 1 Attend A Complementary Training Before Using The Microscope All future users
Operating Instructions for Zeiss LSM 510 Location: GNL 6.312q (BSL3) Questions? Contact: Maxim Ivannikov, maivanni@utmb.edu 1 Attend A Complementary Training Before Using The Microscope All future users
OPERATING INSTRUCTIONS
 Zeiss LSM 510 M eta Confocal M icroscope OPERATING INSTRUCTIONS Starting the System: 1. Turn the black knob on the laser box one-quarter turn from Off to On. You will hear the laser cooling mechanisms
Zeiss LSM 510 M eta Confocal M icroscope OPERATING INSTRUCTIONS Starting the System: 1. Turn the black knob on the laser box one-quarter turn from Off to On. You will hear the laser cooling mechanisms
Boulevard du Temple Daguerrotype (Paris,1838) a busy street? Nyquist sampling for movement
 Boulevard du Temple Daguerrotype (Paris,1838) a busy street? Nyquist sampling for movement CONFOCAL MICROSCOPY BioVis Uppsala, 2017 Jeremy Adler Matyas Molnar Dirk Pacholsky Widefield & Confocal Microscopy
Boulevard du Temple Daguerrotype (Paris,1838) a busy street? Nyquist sampling for movement CONFOCAL MICROSCOPY BioVis Uppsala, 2017 Jeremy Adler Matyas Molnar Dirk Pacholsky Widefield & Confocal Microscopy
Supplemental Figure 1: Histogram of 63x Objective Lens z axis Calculated Resolutions. Results from the MetroloJ z axis fits for 5 beads from each
 Supplemental Figure 1: Histogram of 63x Objective Lens z axis Calculated Resolutions. Results from the MetroloJ z axis fits for 5 beads from each lens with a 1 Airy unit pinhole setting. Many water lenses
Supplemental Figure 1: Histogram of 63x Objective Lens z axis Calculated Resolutions. Results from the MetroloJ z axis fits for 5 beads from each lens with a 1 Airy unit pinhole setting. Many water lenses
Microscope Confocal LSM510 META
 Microscope Confocal LSM510 META Welcome to the Zeiss LSM 510 Meta Confocal tutorial. Before using the LSM 510 META, Log off any other computer that is open with your personal login. You will need to put
Microscope Confocal LSM510 META Welcome to the Zeiss LSM 510 Meta Confocal tutorial. Before using the LSM 510 META, Log off any other computer that is open with your personal login. You will need to put
長庚大學共軛焦顯微鏡課程 長庚大學共軛焦顯微鏡課程. Spot light 長庚大學
 長庚大學共軛焦顯微鏡課程 Spot light 長庚大學共軛焦顯微鏡課程 20071030 長庚大學 Basic principle of Laser Scanning Confocal Microscopy The application of LSM 510 META detector Multiphoton microscopy basic principle and introduction
長庚大學共軛焦顯微鏡課程 Spot light 長庚大學共軛焦顯微鏡課程 20071030 長庚大學 Basic principle of Laser Scanning Confocal Microscopy The application of LSM 510 META detector Multiphoton microscopy basic principle and introduction
ADVANCED METHODS FOR CONFOCAL MICROSCOPY II. Jean-Yves Chatton Sept. 2006
 ADVANCED METHODS FOR CONFOCAL MICROSCOPY II Jean-Yves Chatton Sept. 2006 Workshop outline Confocal microscopy of living cells and tissues X-Z scanning Time series Bleach: FRAP, photoactivation Emission
ADVANCED METHODS FOR CONFOCAL MICROSCOPY II Jean-Yves Chatton Sept. 2006 Workshop outline Confocal microscopy of living cells and tissues X-Z scanning Time series Bleach: FRAP, photoactivation Emission
Leica Sp5 II Confocal User Guide
 Leica Sp5 II Confocal User Guide Turning on the Confocal System (instructions are posted in the room) 1. Turn on Laser Power Button 2. Turn Key to On position 3. Turn on Scanner Power Button 4. Turn on
Leica Sp5 II Confocal User Guide Turning on the Confocal System (instructions are posted in the room) 1. Turn on Laser Power Button 2. Turn Key to On position 3. Turn on Scanner Power Button 4. Turn on
IC 2 S High Performance Objectives
 M i c r o s c o p y f r o m C a r l Z e i s s IC 2 S igh Performance Objectives for Biomedical Applications with Laser Based Imaging Systems LSM,, ConfoCor, TIRF and ELYRA Carl Zeiss offers a large range
M i c r o s c o p y f r o m C a r l Z e i s s IC 2 S igh Performance Objectives for Biomedical Applications with Laser Based Imaging Systems LSM,, ConfoCor, TIRF and ELYRA Carl Zeiss offers a large range
ZEISS LSM 710 NLO Multiphoton microscope Manual/Quick guide
 ZEISS LSM 710 NLO Multiphoton microscope Manual/Quick guide Matyas Molnar, Biovis 2016 Starting the microscpe 1. Check the microscope if everything looks clean and normal. If not, report it in the logbook.
ZEISS LSM 710 NLO Multiphoton microscope Manual/Quick guide Matyas Molnar, Biovis 2016 Starting the microscpe 1. Check the microscope if everything looks clean and normal. If not, report it in the logbook.
Bi/BE 227 Winter Assignment #3. Adding the third dimension: 3D Confocal Imaging
 Bi/BE 227 Winter 2016 Assignment #3 Adding the third dimension: 3D Confocal Imaging Schedule: Jan 20: Assignment Jan 20-Feb 8: Work on assignment Feb 10: Student PowerPoint presentations. Goals for this
Bi/BE 227 Winter 2016 Assignment #3 Adding the third dimension: 3D Confocal Imaging Schedule: Jan 20: Assignment Jan 20-Feb 8: Work on assignment Feb 10: Student PowerPoint presentations. Goals for this
LSM 510 META in Chang Gung University
 Content LSM 510 META in Chang ung University LSM 510 META 路 理 The features and applications of LSM 510 META 01-09 Introduction of the hardware 10-12 Fluorescence observation in conventional microscope
Content LSM 510 META in Chang ung University LSM 510 META 路 理 The features and applications of LSM 510 META 01-09 Introduction of the hardware 10-12 Fluorescence observation in conventional microscope
Zeiss LSM880 Operating Instructions. UTMB Optical Microscopy Core Jan. 16, 2018
 Zeiss LSM880 Operating Instructions UTMB Optical Microscopy Core Jan. 16, 2018 1 1. Power up the microscope Sing the LOGBOOK Steps below will provide power to the computer and all of the microscope components.
Zeiss LSM880 Operating Instructions UTMB Optical Microscopy Core Jan. 16, 2018 1 1. Power up the microscope Sing the LOGBOOK Steps below will provide power to the computer and all of the microscope components.
Operation Guide for the Leica SP2 Confocal Microscope Bio-Imaging Facility Hunter College October 2009
 Operation Guide for the Leica SP2 Confocal Microscope Bio-Imaging Facility Hunter College October 2009 Introduction of Fluoresence Confocal Microscopy The first confocal microscope was invented by Princeton
Operation Guide for the Leica SP2 Confocal Microscope Bio-Imaging Facility Hunter College October 2009 Introduction of Fluoresence Confocal Microscopy The first confocal microscope was invented by Princeton
Fast, high-contrast imaging of animal development with scanned light sheet based structured-illumination microscopy
 nature methods Fast, high-contrast imaging of animal development with scanned light sheet based structured-illumination microscopy Philipp J Keller, Annette D Schmidt, Anthony Santella, Khaled Khairy,
nature methods Fast, high-contrast imaging of animal development with scanned light sheet based structured-illumination microscopy Philipp J Keller, Annette D Schmidt, Anthony Santella, Khaled Khairy,
Confocal imaging on the Leica TCS SP8. 1) Turn the system on. 2) Use TCS user account. 3) Start LAS X software:
 Confocal imaging on the Leica TCS SP8 1) Turn the system on. 2) Use TCS user account. 3) Start LAS X software: 4) Do not touch the microscope while the software is initializing. Choose your options: Turn
Confocal imaging on the Leica TCS SP8 1) Turn the system on. 2) Use TCS user account. 3) Start LAS X software: 4) Do not touch the microscope while the software is initializing. Choose your options: Turn
Structural, optical, and electrical properties of phasecontrolled cesium lead iodide nanowires
 Electronic Supplementary Material Structural, optical, and electrical properties of phasecontrolled cesium lead iodide nanowires Minliang Lai 1, Qiao Kong 1, Connor G. Bischak 1, Yi Yu 1,2, Letian Dou
Electronic Supplementary Material Structural, optical, and electrical properties of phasecontrolled cesium lead iodide nanowires Minliang Lai 1, Qiao Kong 1, Connor G. Bischak 1, Yi Yu 1,2, Letian Dou
Quick Guide for Zeiss 710 Laser Scanning Confocal MGH Cancer Center
 Quick Guide for Zeiss 710 Laser Scanning Confocal MGH Cancer Center For any questions or concerns, please contact: Linda Nieman lnieman@mgh.harvard.edu Office: (617) 643-9684 Cell: (512) 565-8076 Chenyue
Quick Guide for Zeiss 710 Laser Scanning Confocal MGH Cancer Center For any questions or concerns, please contact: Linda Nieman lnieman@mgh.harvard.edu Office: (617) 643-9684 Cell: (512) 565-8076 Chenyue
Zeiss 880 Training Notes Zen 2.3
 Zeiss 880 Training Notes Zen 2.3 1 Turn on the HXP 120V Lamp 2 Turn on Main Power Switch Turn on the Systems PC Switch Turn on the Components Switch. 3 4 5 Turn on the PC and log into your account. Start
Zeiss 880 Training Notes Zen 2.3 1 Turn on the HXP 120V Lamp 2 Turn on Main Power Switch Turn on the Systems PC Switch Turn on the Components Switch. 3 4 5 Turn on the PC and log into your account. Start
Quick Guide for Zeiss 710 Laser Scanning Confocal MGH Cancer Center
 Quick Guide for Zeiss 710 Laser Scanning Confocal MGH Cancer Center For any questions or concerns, please contact: Linda Nieman lnieman@mgh.harvard.edu Office: (617) 643-9684 Cell: (512) 565-8076 Chenyue
Quick Guide for Zeiss 710 Laser Scanning Confocal MGH Cancer Center For any questions or concerns, please contact: Linda Nieman lnieman@mgh.harvard.edu Office: (617) 643-9684 Cell: (512) 565-8076 Chenyue
1 Co Localization and Working flow with the lsm700
 1 Co Localization and Working flow with the lsm700 Samples -1 slide = mousse intestine, Dapi / Ki 67 with Cy3/ BrDU with alexa 488. -1 slide = mousse intestine, Dapi / Ki 67 with Cy3/ no BrDU (but with
1 Co Localization and Working flow with the lsm700 Samples -1 slide = mousse intestine, Dapi / Ki 67 with Cy3/ BrDU with alexa 488. -1 slide = mousse intestine, Dapi / Ki 67 with Cy3/ no BrDU (but with
LSM 800 Confocal Microscope Standard Operation Protocol
 LSM 800 Confocal Microscope Standard Operation Protocol Turning on the system 1. Switch on the Main switch (labeled 1 and 2 ) mounted on the wall. 2. Turn the Laser Key (labeled 3 ) 90 clockwise for power
LSM 800 Confocal Microscope Standard Operation Protocol Turning on the system 1. Switch on the Main switch (labeled 1 and 2 ) mounted on the wall. 2. Turn the Laser Key (labeled 3 ) 90 clockwise for power
Examination, TEN1, in courses SK2500/SK2501, Physics of Biomedical Microscopy,
 KTH Applied Physics Examination, TEN1, in courses SK2500/SK2501, Physics of Biomedical Microscopy, 2009-06-05, 8-13, FB51 Allowed aids: Compendium Imaging Physics (handed out) Compendium Light Microscopy
KTH Applied Physics Examination, TEN1, in courses SK2500/SK2501, Physics of Biomedical Microscopy, 2009-06-05, 8-13, FB51 Allowed aids: Compendium Imaging Physics (handed out) Compendium Light Microscopy
DIC Imaging using Laser Scanning Microscopes (LSM) on Inverted Stands
 DIC Imaging using Laser Scanning Microscopes (LSM) on Inverted Stands Differential Interference Contrast (DIC) imaging is a technique used to increase contrast in brightfield images. In confocal systems,
DIC Imaging using Laser Scanning Microscopes (LSM) on Inverted Stands Differential Interference Contrast (DIC) imaging is a technique used to increase contrast in brightfield images. In confocal systems,
Zeiss 780 Training Notes
 Zeiss 780 Training Notes Turn on Main Switch, System PC and Components Switches 780 Start up sequence Do you need the argon laser (458, 488, 514 nm lines)? Yes Turn on the laser s main power switch and
Zeiss 780 Training Notes Turn on Main Switch, System PC and Components Switches 780 Start up sequence Do you need the argon laser (458, 488, 514 nm lines)? Yes Turn on the laser s main power switch and
Zeiss LSM 510 Multiphoton Confocal Microscope
 Zeiss LSM 510 Multiphoton Confocal Microscope Quick Start User Guide LSU Health Sciences Research Core Facility Table of Contents 1 Safety... Page 3 2 Turn On the System... Page 4 3 Start Up the ZEN Software.
Zeiss LSM 510 Multiphoton Confocal Microscope Quick Start User Guide LSU Health Sciences Research Core Facility Table of Contents 1 Safety... Page 3 2 Turn On the System... Page 4 3 Start Up the ZEN Software.
Contents. Introduction
 Contents Page Contents... 1 Introduction... 1 Starting the System... 2 Introduction to ZEN Efficient Navigation... 5 Setting up the microscope... 10 Configuring the beam path and lasers... 12 Scanning
Contents Page Contents... 1 Introduction... 1 Starting the System... 2 Introduction to ZEN Efficient Navigation... 5 Setting up the microscope... 10 Configuring the beam path and lasers... 12 Scanning
Zeiss LSM 510 Multiphoton Confocal Microscope
 Zeiss LSM 510 Multiphoton Confocal Microscope User Guide LSU Health Sciences Center-Shreveport Research Core Facility Table of Contents 1 Safety... Page 3 2 Turn On the System... Page 4 3 Start Up the
Zeiss LSM 510 Multiphoton Confocal Microscope User Guide LSU Health Sciences Center-Shreveport Research Core Facility Table of Contents 1 Safety... Page 3 2 Turn On the System... Page 4 3 Start Up the
SUPPLEMENTAL MATERIAL
 SUPPLEMENTAL MATERIAL 1 - Folios and areas of analysis Figure S1.1. Folio 4, areas of analysis for microxrf ( ), FORS ( ), micro-samples for Raman and FTIR ( ) and Raman in-situ ( ). Figure S1.2. Folio
SUPPLEMENTAL MATERIAL 1 - Folios and areas of analysis Figure S1.1. Folio 4, areas of analysis for microxrf ( ), FORS ( ), micro-samples for Raman and FTIR ( ) and Raman in-situ ( ). Figure S1.2. Folio
Trans-illumination a Solution for Excitation of GFP Transfected Plants in Petri Dishes for In Vivo Imaging
 Trans-illumination a Solution for Excitation of GFP Transfected Plants in Petri Dishes for In Vivo Imaging Manfred Hennecke 1 Abstract This application note aims to show excitation of GFP-transfected plants
Trans-illumination a Solution for Excitation of GFP Transfected Plants in Petri Dishes for In Vivo Imaging Manfred Hennecke 1 Abstract This application note aims to show excitation of GFP-transfected plants
3. are adherent cells (ie. cells in suspension are too far away from the coverslip)
 Before you begin, make sure your sample... 1. is seeded on #1.5 coverglass (thickness = 0.17) 2. is an aqueous solution (ie. fixed samples mounted on a slide will not work - not enough difference in refractive
Before you begin, make sure your sample... 1. is seeded on #1.5 coverglass (thickness = 0.17) 2. is an aqueous solution (ie. fixed samples mounted on a slide will not work - not enough difference in refractive
Leica SP8 TCS Users Manual
 Version : 07/08/0 Leica SP8 TCS Users Manual Start up:. Turn the PC Microscope, Scanner Power, Laser Power, and the Laser Emission key to on (bottom right of desk).. Turn on the fluorescent lamp (top left
Version : 07/08/0 Leica SP8 TCS Users Manual Start up:. Turn the PC Microscope, Scanner Power, Laser Power, and the Laser Emission key to on (bottom right of desk).. Turn on the fluorescent lamp (top left
Microscopic Structures
 Microscopic Structures Image Analysis Metal, 3D Image (Red-Green) The microscopic methods range from dark field / bright field microscopy through polarisation- and inverse microscopy to techniques like
Microscopic Structures Image Analysis Metal, 3D Image (Red-Green) The microscopic methods range from dark field / bright field microscopy through polarisation- and inverse microscopy to techniques like
Renishaw InVia Raman microscope
 Laser Spectroscopy Labs Renishaw InVia Raman microscope Operation instructions 1. Turn On the power switch, system power switch is located towards the back of the system on the right hand side. Wait ~10
Laser Spectroscopy Labs Renishaw InVia Raman microscope Operation instructions 1. Turn On the power switch, system power switch is located towards the back of the system on the right hand side. Wait ~10
Leica SP8 TCS Users Manual
 Leica SP8 TCS Users Manual Follow the procedure for start up and log on as posted in the lab. Please log on with your account only and do not share your password with anyone. We track and confirm usage
Leica SP8 TCS Users Manual Follow the procedure for start up and log on as posted in the lab. Please log on with your account only and do not share your password with anyone. We track and confirm usage
NOVEL APPLICATIONS OF CONFOCAL MICROSCOPY TECHNIQUES IN COATINGS RESEARCH
 ARKEMA COATING RESINS NOVEL APPLICATIONS OF CONFOCAL MICROSCOPY TECHNIQUES IN COATINGS RESEARCH DOUG MALL FOR DR. WENJUN WU 9/20/2018 Wood Coatings & Substrates Conference 2018 OUTLINE Introduction Confocal
ARKEMA COATING RESINS NOVEL APPLICATIONS OF CONFOCAL MICROSCOPY TECHNIQUES IN COATINGS RESEARCH DOUG MALL FOR DR. WENJUN WU 9/20/2018 Wood Coatings & Substrates Conference 2018 OUTLINE Introduction Confocal
Electrical and Optical Tunability in All-Inorganic Halide. Perovskite Alloy Nanowires
 Supporting Information for: Electrical and Optical Tunability in All-Inorganic Halide Perovskite Alloy Nanowires Teng Lei, 1 Minliang Lai, 1 Qiao Kong, 1 Dylan Lu, 1 Woochul Lee, 2 Letian Dou, 3 Vincent
Supporting Information for: Electrical and Optical Tunability in All-Inorganic Halide Perovskite Alloy Nanowires Teng Lei, 1 Minliang Lai, 1 Qiao Kong, 1 Dylan Lu, 1 Woochul Lee, 2 Letian Dou, 3 Vincent
Travel to New Dimensions- LSM 880. The Resolution of a Microscope is limited. The Resolution of a Microscope is limited. Image. Image. Object.
 Travel to New Dimensions- LSM 880 LSM 880: The Power of Sensitivity Our Latest Member of the LSM 880 with GaAsP Detectors Sensitivity, and Ease of Use Innovative High-End Laser Scanning Microscopes from
Travel to New Dimensions- LSM 880 LSM 880: The Power of Sensitivity Our Latest Member of the LSM 880 with GaAsP Detectors Sensitivity, and Ease of Use Innovative High-End Laser Scanning Microscopes from
Imaging Beyond the Basics: Optimizing Settings on the Leica SP8 Confocal
 Imaging Beyond the Basics: Optimizing Settings on the Leica SP8 Confocal Todays Goal: Introduce some additional functionalities of the Leica SP8 confocal HyD vs. PMT detectors Dye Assistant Scanning By
Imaging Beyond the Basics: Optimizing Settings on the Leica SP8 Confocal Todays Goal: Introduce some additional functionalities of the Leica SP8 confocal HyD vs. PMT detectors Dye Assistant Scanning By
Zeiss Axiovert 135 Fluorescence Microscope Quick Guide / Operations Manual (v. 1.0 February 09)
 University of Chicago Integrated Light Microscopy Core Dr. Vytas Bindokas, Director http://digital.bsd.uchicago.edu By: Christine Labno, Assistant Director Room: AB-129 Phone: 4-9040 Zeiss Axiovert 135
University of Chicago Integrated Light Microscopy Core Dr. Vytas Bindokas, Director http://digital.bsd.uchicago.edu By: Christine Labno, Assistant Director Room: AB-129 Phone: 4-9040 Zeiss Axiovert 135
3 Choose the Channels button and set the Channel Settings. Set the Pinhole to 1 Airy unit.
 1 Set up the confocal light path for imaging a green dye (e.g. Alexa488-EGFP). For example, under the Configuration Control window the light path could be set up as shown here using the 488 nm LASER (found
1 Set up the confocal light path for imaging a green dye (e.g. Alexa488-EGFP). For example, under the Configuration Control window the light path could be set up as shown here using the 488 nm LASER (found
Imaging Changes in Cytoplasmic Calcium Using the Yellow Cameleon 3.6 Biosensor and Confocal Microscopy. Sarah J. Swanson and Simon Gilroy
 Chapter 27 Imaging Changes in Cytoplasmic Calcium Using the Yellow Cameleon 3.6 Biosensor and Confocal Microscopy Sarah J. Swanson and Simon Gilroy Abstract Changes in the concentration of cytoplasmic
Chapter 27 Imaging Changes in Cytoplasmic Calcium Using the Yellow Cameleon 3.6 Biosensor and Confocal Microscopy Sarah J. Swanson and Simon Gilroy Abstract Changes in the concentration of cytoplasmic
Training Guide for Leica SP8 Confocal/Multiphoton Microscope
 Training Guide for Leica SP8 Confocal/Multiphoton Microscope LAS AF v3.3 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Turn ON power switch for epifluorescence
Training Guide for Leica SP8 Confocal/Multiphoton Microscope LAS AF v3.3 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Turn ON power switch for epifluorescence
MIF ZEISS LSM510 CONFOCAL USER PROTOCOL
 MIF ZEISS LSM510 CONFOCAL USER PROTOCOL START-UP Turn on the Mercury Bulb Power Supply (if needed). Power-on the Control Box. Turn on the computer. Open the LSM 510 software. Choose Scan New Images and
MIF ZEISS LSM510 CONFOCAL USER PROTOCOL START-UP Turn on the Mercury Bulb Power Supply (if needed). Power-on the Control Box. Turn on the computer. Open the LSM 510 software. Choose Scan New Images and
Precision-tracking of individual particles By Fluorescence Photo activation Localization Microscopy(FPALM) Presented by Aung K.
 Precision-tracking of individual particles By Fluorescence Photo activation Localization Microscopy(FPALM) Presented by Aung K. Soe This FPALM research was done by Assistant Professor Sam Hess, physics
Precision-tracking of individual particles By Fluorescence Photo activation Localization Microscopy(FPALM) Presented by Aung K. Soe This FPALM research was done by Assistant Professor Sam Hess, physics
Opterra II Multipoint Scanning Confocal Microscope. Innovation with Integrity
 Opterra II Multipoint Scanning Confocal Microscope Enabling 4D Live-Cell Fluorescence Imaging through Speed, Sensitivity, Viability and Simplicity Innovation with Integrity Fluorescence Microscopy The
Opterra II Multipoint Scanning Confocal Microscope Enabling 4D Live-Cell Fluorescence Imaging through Speed, Sensitivity, Viability and Simplicity Innovation with Integrity Fluorescence Microscopy The
Application Note. The New 2D Superresolution Mode for ZEISS Airyscan 120 nm Lateral Resolution without Acquiring a Z-stack
 The New 2D Superresolution Mode for ZEISS Airyscan 120 nm Lateral Resolution without Acquiring a Z-stack The New 2D Superresolution Mode for ZEISS Airyscan 120 nm Lateral Resolution without Acquiring a
The New 2D Superresolution Mode for ZEISS Airyscan 120 nm Lateral Resolution without Acquiring a Z-stack The New 2D Superresolution Mode for ZEISS Airyscan 120 nm Lateral Resolution without Acquiring a
7 CHAPTER 7: REFRACTIVE INDEX MEASUREMENTS WITH COMMON PATH PHASE SENSITIVE FDOCT SETUP
 7 CHAPTER 7: REFRACTIVE INDEX MEASUREMENTS WITH COMMON PATH PHASE SENSITIVE FDOCT SETUP Abstract: In this chapter we describe the use of a common path phase sensitive FDOCT set up. The phase measurements
7 CHAPTER 7: REFRACTIVE INDEX MEASUREMENTS WITH COMMON PATH PHASE SENSITIVE FDOCT SETUP Abstract: In this chapter we describe the use of a common path phase sensitive FDOCT set up. The phase measurements
Sensitive imaging of spectrally overlapping fluorochromes using the LSM 510 META
 Invited Paper Sensitive imaging of spectrally overlapping fluorochromes using the LSM 510 META Mary E. Dickinson a*, Christopher W. Waters a, Gregory Bearman b, Ralf Wolleschensky c, Sebastian Tille d
Invited Paper Sensitive imaging of spectrally overlapping fluorochromes using the LSM 510 META Mary E. Dickinson a*, Christopher W. Waters a, Gregory Bearman b, Ralf Wolleschensky c, Sebastian Tille d
Training Guide for Carl Zeiss LSM 880 with AiryScan FAST
 Training Guide for Carl Zeiss LSM 880 with AiryScan FAST ZEN 2.3 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2018) Power ON Routine 1 2 Turn ON Main Switch from the remote control
Training Guide for Carl Zeiss LSM 880 with AiryScan FAST ZEN 2.3 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2018) Power ON Routine 1 2 Turn ON Main Switch from the remote control
Microscope Confocal Sp2 Upright.
 Microscope Confocal Sp2 Upright. Welcome to the Leica Sp2 Confocal Upright tutorial. Before using the Sp2 Invert, You will need to put down your name on the reservation system = http://svintranet.epfl.ch/index.php?optio
Microscope Confocal Sp2 Upright. Welcome to the Leica Sp2 Confocal Upright tutorial. Before using the Sp2 Invert, You will need to put down your name on the reservation system = http://svintranet.epfl.ch/index.php?optio
Quick Start Guide. Leica SP5 X
 Quick Start Guide Leica SP5 X Please note: Some of the information in this guide was taken from Leica Microsystems Leica TCS SP5 LAS AF Guide for New Users. This work is licensed under the Creative Commons
Quick Start Guide Leica SP5 X Please note: Some of the information in this guide was taken from Leica Microsystems Leica TCS SP5 LAS AF Guide for New Users. This work is licensed under the Creative Commons
b. Turn the power switch and key to on position for blue laser.
 OLYMPUS FLUOVIEW 300 CONFOCAL MICOSCOPE OPERATION PROCEDURE 1. Turn ON microscope in this order: 1) Turn on mercury lamp (Note: once the mercury lamp is turned off, DO NOT turn it back on for at least
OLYMPUS FLUOVIEW 300 CONFOCAL MICOSCOPE OPERATION PROCEDURE 1. Turn ON microscope in this order: 1) Turn on mercury lamp (Note: once the mercury lamp is turned off, DO NOT turn it back on for at least
Light Microscopy. Upon completion of this lecture, the student should be able to:
 Light Light microscopy is based on the interaction of light and tissue components and can be used to study tissue features. Upon completion of this lecture, the student should be able to: 1- Explain the
Light Light microscopy is based on the interaction of light and tissue components and can be used to study tissue features. Upon completion of this lecture, the student should be able to: 1- Explain the
Figure S1 Figure S1. Wild type IgG and FcRn colocalize in APPL+ TCs.
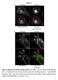 Figure S1 Figure S1. Wild type IgG and FcRn colocalize in APPL+ TCs. HMEC-1 cells were cotransfected with GFP-FcRn and β2m. Transfected cells were incubated with 200 μg/ml Alexa 647-wild type IgG (A) or
Figure S1 Figure S1. Wild type IgG and FcRn colocalize in APPL+ TCs. HMEC-1 cells were cotransfected with GFP-FcRn and β2m. Transfected cells were incubated with 200 μg/ml Alexa 647-wild type IgG (A) or
Leica TCS SL Confocal Training. Neuroscience Imaging Core Staff. Core Director. Facility Manager
 Leica TCS SL Confocal Training Neuroscience Imaging Facility The Ohio State University Rightmire Hall 614-292-1367 Staff Core Director Anthony Brown, Ph. D. 060 Rightmire Hall 614-292-1205 brown.2302@osu.edu
Leica TCS SL Confocal Training Neuroscience Imaging Facility The Ohio State University Rightmire Hall 614-292-1367 Staff Core Director Anthony Brown, Ph. D. 060 Rightmire Hall 614-292-1205 brown.2302@osu.edu
Opterra. Multipoint Scanning Confocal Microscope. Innovation with Integrity. Cell-Friendly, High-Speed, High-Resolution Imaging
 Opterra Multipoint Scanning Confocal Microscope Cell-Friendly, High-Speed, High-Resolution Imaging Innovation with Integrity Fluorescence Microscopy Opterra Multipoint Scanning Confocal Microscope Superior
Opterra Multipoint Scanning Confocal Microscope Cell-Friendly, High-Speed, High-Resolution Imaging Innovation with Integrity Fluorescence Microscopy Opterra Multipoint Scanning Confocal Microscope Superior
Scanning Ion Conductance Microscope ICnano
 Sperm Cell Epithelial Cells I nner Ear Hair Cells I nner Ear Hair Cell Neurons E- Coli Bac teria Scanning Ion Conductance Microscope ICnano About ionscope About ionscope The ionscope scanning ion conductance
Sperm Cell Epithelial Cells I nner Ear Hair Cells I nner Ear Hair Cell Neurons E- Coli Bac teria Scanning Ion Conductance Microscope ICnano About ionscope About ionscope The ionscope scanning ion conductance
Multi-channel imaging cytometry with a single detector
 Multi-channel imaging cytometry with a single detector Sarah Locknar 1, John Barton 1, Mark Entwistle 2, Gary Carver 1 and Robert Johnson 1 1 Omega Optical, Brattleboro, VT 05301 2 Philadelphia Lightwave,
Multi-channel imaging cytometry with a single detector Sarah Locknar 1, John Barton 1, Mark Entwistle 2, Gary Carver 1 and Robert Johnson 1 1 Omega Optical, Brattleboro, VT 05301 2 Philadelphia Lightwave,
Microscopy from Carl Zeiss LSM 700. Laser Scanning Microscope. High-End for All Uncompromised Quality and Operating Convenience
 Microscopy from Carl Zeiss LSM 700 Laser Scanning Microscope High-End for All Uncompromised Quality and Operating Convenience 2 3 The New LSM 700 from Carl Zeiss From a specialists system to the high-end
Microscopy from Carl Zeiss LSM 700 Laser Scanning Microscope High-End for All Uncompromised Quality and Operating Convenience 2 3 The New LSM 700 from Carl Zeiss From a specialists system to the high-end
The Zeiss AiryScan System, Confocal Four.
 The Zeiss AiryScan System, Confocal Four. Overview. The Zeiss AiryScan module is a segmented, radially stacked GaASP detector and collector system designed to subsample the airy disk of a point emission
The Zeiss AiryScan System, Confocal Four. Overview. The Zeiss AiryScan module is a segmented, radially stacked GaASP detector and collector system designed to subsample the airy disk of a point emission
Fabry Perot Resonator (CA-1140)
 Fabry Perot Resonator (CA-1140) The open frame Fabry Perot kit CA-1140 was designed for demonstration and investigation of characteristics like resonance, free spectral range and finesse of a resonator.
Fabry Perot Resonator (CA-1140) The open frame Fabry Perot kit CA-1140 was designed for demonstration and investigation of characteristics like resonance, free spectral range and finesse of a resonator.
(Quantitative Imaging for) Colocalisation Analysis
 (Quantitative Imaging for) Colocalisation Analysis or Why Colour Merge / Overlay Images are EVIL! Special course for DIGS-BB PhD program What is an Image anyway..? An image is a representation of reality
(Quantitative Imaging for) Colocalisation Analysis or Why Colour Merge / Overlay Images are EVIL! Special course for DIGS-BB PhD program What is an Image anyway..? An image is a representation of reality
Confocal Microscopy. Kristin Jensen
 Confocal Microscopy Kristin Jensen 17.11.05 References Cell Biological Applications of Confocal Microscopy, Brian Matsumoto, chapter 1 Studying protein dynamics in living cells,, Jennifer Lippincott-Schwartz
Confocal Microscopy Kristin Jensen 17.11.05 References Cell Biological Applications of Confocal Microscopy, Brian Matsumoto, chapter 1 Studying protein dynamics in living cells,, Jennifer Lippincott-Schwartz
Shreyash Tandon M.S. III Year
 Shreyash Tandon M.S. III Year 20091015 Confocal microscopy is a powerful tool for generating high-resolution images and 3-D reconstructions of a specimen by using point illumination and a spatial pinhole
Shreyash Tandon M.S. III Year 20091015 Confocal microscopy is a powerful tool for generating high-resolution images and 3-D reconstructions of a specimen by using point illumination and a spatial pinhole
Development of a spectrometry system Using lock-in amplification technique
 VNU. JOURNAL OF SCIENCE, Mathematics - Physics, T.xXI, n 0 2, 2005 Development of a spectrometry system Using lock-in amplification technique Department of Physics, College of Science, VNU Abstract. Raman
VNU. JOURNAL OF SCIENCE, Mathematics - Physics, T.xXI, n 0 2, 2005 Development of a spectrometry system Using lock-in amplification technique Department of Physics, College of Science, VNU Abstract. Raman
3D light microscopy techniques
 3D light microscopy techniques The image of a point is a 3D feature In-focus image Out-of-focus image The image of a point is not a point Point Spread Function (PSF) 1D imaging 1 1 2! NA = 0.5! NA 2D imaging
3D light microscopy techniques The image of a point is a 3D feature In-focus image Out-of-focus image The image of a point is not a point Point Spread Function (PSF) 1D imaging 1 1 2! NA = 0.5! NA 2D imaging
Statistical Evaluation of Confocal Microscopy Images
 Published 2001 Wiley-Liss, Inc. Cytometry 44:295 308 (2001) Statistical Evaluation of Confocal Microscopy Images Robert M. Zucker* and Owen T. Price Reproductive Toxicology Division, National Health and
Published 2001 Wiley-Liss, Inc. Cytometry 44:295 308 (2001) Statistical Evaluation of Confocal Microscopy Images Robert M. Zucker* and Owen T. Price Reproductive Toxicology Division, National Health and
Technology Note ZEISS LSM 880 with Airyscan
 Technology Note ZEISS LSM 880 with Airyscan Introducing the Fast Acquisition Mode ZEISS LSM 880 with Airyscan Introducing the Fast Acquisition Mode Author: Dr. Annette Bergter Carl Zeiss Microscopy GmbH,
Technology Note ZEISS LSM 880 with Airyscan Introducing the Fast Acquisition Mode ZEISS LSM 880 with Airyscan Introducing the Fast Acquisition Mode Author: Dr. Annette Bergter Carl Zeiss Microscopy GmbH,
Study on the Binder Distribution related to Drying
 International Symposium on Computers & Informatics (ISCI 2015) Study on the Binder Distribution related to Drying Ying Li 1,a, Qinming Wang 1, Wenjuan Gu 1 and Banggui He 1 1 Faculty of Mechanical and
International Symposium on Computers & Informatics (ISCI 2015) Study on the Binder Distribution related to Drying Ying Li 1,a, Qinming Wang 1, Wenjuan Gu 1 and Banggui He 1 1 Faculty of Mechanical and
DIC Imaging using Laser Scanning Microscopes (LSMs) on Axio Imager Stands
 DIC Imaging using Laser Scanning Microscopes (LSMs) on Axio Imager Stands Differential Interference Contrast (DIC) imaging is a technique used to increase contrast in brightfield images. In confocal systems,
DIC Imaging using Laser Scanning Microscopes (LSMs) on Axio Imager Stands Differential Interference Contrast (DIC) imaging is a technique used to increase contrast in brightfield images. In confocal systems,
Guide to Confocal 5. Starting session
 Guide to Confocal 5 Remember that when booking and before starting session you can check for any problems at https://www.bris.ac.uk/biochemistry/uobonly/cif/index.html Starting session Switch on microscope
Guide to Confocal 5 Remember that when booking and before starting session you can check for any problems at https://www.bris.ac.uk/biochemistry/uobonly/cif/index.html Starting session Switch on microscope
TRAINING MANUAL. Olympus FV1000
 TRAINING MANUAL Olympus FV1000 September 2014 TABLE OF CONTENTS A. Start-Up Procedure... 1 B. Visual Observation under the Microscope... 1 C. Image Acquisition... 4 A brief Overview of the Settings...
TRAINING MANUAL Olympus FV1000 September 2014 TABLE OF CONTENTS A. Start-Up Procedure... 1 B. Visual Observation under the Microscope... 1 C. Image Acquisition... 4 A brief Overview of the Settings...
Chemical Imaging. Whiskbroom Imaging. Staring Imaging. Pushbroom Imaging. Whiskbroom. Staring. Pushbroom
 Chemical Imaging Whiskbroom Chemical Imaging (CI) combines different technologies like optical microscopy, digital imaging and molecular spectroscopy in combination with multivariate data analysis methods.
Chemical Imaging Whiskbroom Chemical Imaging (CI) combines different technologies like optical microscopy, digital imaging and molecular spectroscopy in combination with multivariate data analysis methods.
Training Guide for Carl Zeiss LSM 5 LIVE Confocal Microscope
 Training Guide for Carl Zeiss LSM 5 LIVE Confocal Microscope AIM 4.2 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Verify that main power switches on the
Training Guide for Carl Zeiss LSM 5 LIVE Confocal Microscope AIM 4.2 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Verify that main power switches on the
