Fluorescence (Luminescence) Lifetime Imaging application simplified... with the pco.flim
|
|
|
- Kristopher Fox
- 6 years ago
- Views:
Transcription
1 Fluorescence (Luminescence) Life Imaging application simplified... with the pco.flim lifes from 100 ps to 100 µs unique resolution 1008 x 1008 pixels high frame rate up to 90 fps frequency synthesizer 5 khz - 40 MHz
2 Fluorescence (Luminescence) Life Imaging PCO.FLIM - FLUORESCENCE LIFETIME IMAGING CMOS CAMERA Content 1 Photoluminescence 3 2 Time Response of Photoluminescence 4 3 Luminescence Intensity & Life 4 4 Luminescence Life & FLIM 5 5 How to do Luminescence Life Imaging Time Domain Frequency Domain 7 6 QMFLIM2 - A Directly Modulatable CMOS Image Sensor 9 7 pco.flim - Camera System for Luminescence Life Imaging Camera System Principle Structure of an Experimental Set-Up Single Frequency Measurement Pixel Asymmetry Fluorescence Standards from Starna Scientific Proper Modulation Frequency Selection Coherent Light and De-Speckling Optimum Optic Coupling of Excitation Light into a Microscope 18 Appendix A1.1 Lock-In Detection and Cross-Correlation Function 19 A1.2 Numerical Harmonic Analysis 20 A1.3 Order of Phase Images Using the pco.flim Camera 21 A1.4 Referencing 23 A1.5 Sampling Theory pco.flim Result Images 24 2
3 Fluorescence (Luminescence) Life Imaging 1 PHOTOLUMINESCENCE Photoluminescence describes an interaction of light and matter, when light is absorbed by certain compounds. With this absorption of energy the molecules enter an excited state. Due to the fact that this excited state is not a stable state, several energy conversions take place. Some of these conversions are radiationless and some of them emit light with a lower energy than the absorbed light. This radiative conversion is called luminescence (see fig. 1). Depending on the pathway of the physical process that causes the light emission the luminescence is either called fluorescence or phosphorescence. The energy states and conversions can be described by an energy level diagram, or Jablonski diagram, which is shown in figure 2. Another option involves an intersystem crossing (fig. 2, ISC) which includes a quantum mechanically less probable reversion of the electron spin and a consecutive emission of light with the energy hν P, which is called phosphorescence. There are also energy transfers possible between the atoms of a molecule, but they are all radiationless and they are excluded from figure 2 for simplicity. All of these conversions require and therefore the radiative conversions like fluorescence and phosphorescence can be characterized and distinguished from each other by their corresponding constants. Generally fluorescence takes less, therefore the corresponding decay s are shorter in the range up to 10-8 s (10 ns). The intersystem crossing is less probable, which means, if it happens, it takes more. Because of that the corresponding decay s are longer in the range of s (μs ms). These different decay s represent a good distinction whether the emitted light can be considered fluorescence or phosphorescence, if the underlying process is unknown. Figure 2 Figure 1: Images of fluorescing plastic slides and a cuvette with a fluorescine solution. After absorption of light with the energy hν A (see fig. 2) the molecule turns from the ground energy level S 0 into an excited state S 1 or S 2 or other. Since these states are unstable the molecule tries to return to the stable ground state. During internal conversions (fig.2, IC) part of the absorbed light energy is converted into vibrational or thermal energy. From there it is possible that a radiationless conversion happens or light is emitted, with the energy hν F, which is called fluorescence. Jablonski diagram, an energy level scheme for photoluminescence. 3
4 Fluorescence (Luminescence) Life Imaging 2 TIME RESPONSE OF PHOTOLUMINESCENCE Assuming the availability of a light source, which rises and descends more than a hundred s faster than the luminescence, then figure 3 shows the temporal behavior of the excitation light and the luminescence that it has caused. When the excitation light is switched on (blue curve in fig. 3) the luminescence emission starts as well, but it takes some until all radiative emission begins, therefore a delay is visible that in technical terms is called a rise of a lowpass system. Then a steady state is reached with a constant luminescence emission and constant excitation (if photo bleaching and other loss effects are neglected). When the excitation light is switched off, a decay behavior can be observed and measured, characterized by the same constant as for the rise curve. It takes some until all excited levels are empty again. This constant is usually called luminescence decay or luminescence life. Together with the intensity (quantum efficiency) it is one of the characteristic parameters of each luminophore, which can be used for analytical or sensing purposes. Figure 3: Temporal behavior of excitation light (blue) and corresponding luminescence (orange) emission (intensity vs. ). 3 LUMINESCENCE INTENSITY & LIFETIME Both parameters, the luminescence intensity and the life, are widely used for a large variety of analytical measurements. In Life Sciences the measurement of the luminescence intensity is a major tool for all kinds of microscopy and analysis, to visualize structures, metabolic processes, etc. If luminescent indicators are applied both parameters could be used for calibration purposes. Somes they contain the same information, but in many applications they are diverse, like in figure 4 where intensity and life distribution show differences. While there are plenty of instruments to detect or measure luminescence images, only few exist to measure the 2D distributions of the corresponding luminescence lifes. In addition, if any luminophore should be used for sensing purposes (e.g. pressure sensitive paint, oxygen sensing) and a calibration curve has to be determined, every intensity based calibration strongly depends on the stability of the light field and the optical situation. In simpler words: For intensity based calibrations it matters whether 100 or 500 molecules are emitting light, but for life based calibrations it is not relevant as long as they all have the same life. Figure 4: Two sets of fluorescence intensity images and their corresponding fluorescence life distributions. Left: HEK-293 cells that expressed DJ-1/ CFP, which was a control experiment. Right: an endogenous fluorescence image of a daisy slice sample and the corresponding life distribution. The left image was recorded with a 60x oil immersion objective and the right image was obtained with 40x air objective. The modulation frequency was 30 MHz and the exposure for one double image was 500 ms (left) and 100 ms (right). The lifes are color coded in units of ns. (courtesy of Prof. Dr. F.S. Wouters and Dr. G. Bunt, University Medicine Göttingen) 4 Authors: Gerhard Holst & Robert Franke PCO AG
5 Fluorescence (Luminescence) Life Imaging 4 LUMINESCENCE LIFETIME & FLIM As shown in chapter 2 the luminescence life is one characteristic parameter of a luminophore, and it is either called a life (describing the, the excited molecules stay or live in an excited state) or decay (the characteristic that describes the luminescence decay curve after switching off of the excitation light). Both expressions are commonly used for the same constant. Furthermore, the photoluminescence is split up into fluorescence and phosphorescence with their corresponding lifes, and in many cases the term fluorescence life is used to name or describe most luminescence life measurements. the term PLIM for phosphorescence imaging, ignoring the origin of the M for microscopy. The literally correct term of LLI or LLIM for Luminescence Life Imaging is not used, due to the simple fact that it does not sound good. Following the rule of common use the new camera system for luminescence life imaging is called pco.flim. When the first luminescence life images were measured, this technique was called Fluorescence Life Imaging Microscopy, because the measurements were made with microscope, so it was abbreviated to FLIM. Later this acronym was used for all types of fluorescence life imaging. Recently some scientists recognize the differences in the underlying processes again and created 5 HOW TO DO LUMINESCENCE LIFETIME IMAGING 5.1 Time Domain In domain, like figure 3 suggests, the ultimate goal is the acquisition of the decay curve. Since the quantum efficiency of photoluminescence is usually rather small, the light intensity of the luminescence signal is weak. Therefore the most common detection methods involve very sensitive detectors like photomultiplier tubes and low signal detection via photon counting. Hence for single point measurements and scanning applications the most common method is Time Correlated Single Photon Counting (TC- SPC). Here many measuring points are collected in the domain, when the excitation light is switched off (see fig. 5). The measuring systems require a large bandwidth, such that they do not alter the decay curve due to any bandwidth limitation. When enough data points are collected, a predefined decay curve based on the assumption of one or more present decay s is fitted. The decay s resulting from this fitting process are the measured data. For imaging purposes every point in the sample is scanned and processed in this way. This method gives good results for multi-exponential decay curves, but it is relatively slow for image uptake due to the involved scanning process. Another option for domain imaging is the synchronized integration over different slots or windows of the decay curve (also called boxcar integration). In this case one image is taken over a certain interval of the decay curve. A second image has to be taken over a different interval of the decay curve (see figure 6). image 1 image 2 Figure 5: Typical TCSPC measurements (dots) with the corresponding fitted decay curves (lines). Figure 6: Integration of luminescence over two different intervals of the decay curve, resulting in two images. 5
6 Fluorescence (Luminescence) Life Imaging 5 HOW TO DO LUMINESCENCE LIFETIME IMAGING If the signal is weak the procedure can be repeated many s while each portion of light generated charge carriers is integrated on the image sensor (sensicam sensimod, pco.1600, 2000, 4000 mod). Thus, in most cases the modulated light signal is synchronized with the image recording so, that the generated charge carriers are only accepted in the externally adjusted interval, which is active only when the excitation light is off. purposes when the luminescence life unambiguously correlates to the change of an analyte, it is possible to simplify the detection scheme by a ratio type measurement called Rapid Life Determination 1. Instead of using two windows in the low light range, the first window is chosen in the rise phase of the luminescence signal and the second integration window is placed in the decay phase of the luminescence signal (see figure 8). Note: Although in principle this type of measurement does not require excitation and emission filters to separate the radiation, they should be used anyway. The excitation light might be significantly brighter than the luminescence, exceeding the blocking efficiency of the image sensor, which integrates during the exposure, but has to block the excitation light during the image sensor off phase. Figure 7 illustrates the real integration on the image sensor for the two images. exposure image 1 image 1 image 2 Figure 8: Integration of luminescence in two images over two different windows of the decay curve for the use of the Rapid Life Determination (RLD) method (intensity vs. ). Instead of evaluating the luminescence life or decay the ratio of both images is used as a measuring signal for calibration of the luminescence signal vs. an analyte concentration. This method has fewer requirements with respect to resolution, and it delivers a simple decay depending signal for measuring purposes. exposure image 2 1 An error analysis of the rapid life determination method for the evaluation of single exponential decays, Richard M. Ballew and J.N. Demas, Analytical Chemistry,1989, Vol. 61 (1), pp Figure 7: Integration of luminescence in two images over two different windows of the decay curve using a real camera. The integration on the chip is repeated numerous s until enough light has been collected for an image readout. In contrast to the single point measurements, using this type of measurement with two images integrated over different windows only a single luminescence life or decay can be resolved. If more lifes are supposed to be present in the imaged sample, more integration windows have to be recorded, otherwise these lifes cannot be resolved. For short lifes and weak signals this detection scheme might require both, short exposure s and long total accumulation s. In case the real life information is not required, but only a stable, reproducible signal is of interest, for example for sensing 6 Authors: Gerhard Holst & Robert Franke PCO AG
7 Fluorescence (Luminescence) Life Imaging 5 HOW TO DO LUMINESCENCE LIFETIME IMAGING 5.2 Frequency Domain In frequency domain a continuously modulated excitation signal is used instead of a pulse or repetitive pulse signal. This requires a good separation of excitation and luminescence light by proper optical filtering. The response of such an excitation is shown in figure 9. With the division of the amplitudes (a exc, a em ) by their constant components (b exc, b em ) a parameter is derived, which is independent of the intensity. It is called the modulation index (m exc, m em ). The relation between the modulation indices and the luminescence life τ M in a mono-exponential decay is as follows: In the ideal case of a maximum modulation depth, the excitation signal changes between maximum light and darkness (zero light). The photoluminescent molecules respond by emitting luminescence. This decay is not instantaneous and has a delay, with a smaller amplitude and changed amplitude. Changes in the delay, changed amplitude and constant component, can be measured and used to determine the luminescence life of the molecule. Due to the known modulation frequency the delay introduced by the luminescent molecules can also be expressed by an equivalent phase angle or phase shift. In the technical world this is important, since luminophores can be considered as linear -invariant (LTI) systems, which can equivalently be analyzed either in the or the frequency domain. Figure 10 shows both the excitation and the luminescence emission signal in one graph and denotes the important characteristic parameters, which can be used for the life determination. modulated excitation aexc bexc Φ aem bem modulated emission Figure 9: Time response of excitation light (blue) and luminescence (orange) emission if luminescence life imaging is done in frequency domain (extract from continuous signals, intensity vs. ). The delay between both sinusoidal signals of the same frequency can be expressed by a phase shift with its corresponding phase angle Φ. This Φ also has a direct relation to the luminescence life τ P in a mono-exponential decay and the modulation frequency: For multi-exponential decays the relationships are more complex 2. If the parameters modulation index and phase angle can be determined at multiple frequencies, it would therefore be possible to derive the corresponding luminescence lifes. Figure 11: Sinusoidal signal (orange) with sampling points (grey circles) For a proper measurement of these parameters, it is necessary to acquire the sinusoidal signal. In digital signal processing this is usually done by sampling and a subsequent reconstruction of the signal. Figure 11 shows a sinusoidal signal with some sample points. For a correct reconstruction it is necessary to sample at more than twice the frequency of the signal (Nyquist criterion), therefore four sample points or more per period should be sufficient. For imaging applications it is not possible to acquire these sample points, since it would require extremely short exposure s with insufficient light, even for a repetition. Hence, instead of a discrete sampling the luminescence signal is integrated over a certain window as illustrated in figure 12. Figure 10: Time responses of excitation light (blue) and luminescence (orange) emission if luminescence life imaging is done in frequency domain (extract from continuous signals). Amplitudes a exc and a em, constant or direct components b exc and b em and phase angle or phase shift F are denoted. 2 See: Principles of Fluorescence Spectroscopy, J.R. Lakowicz, Springer or Hochintegriertes Kamerasystem für die Multifrequenz-Lumineszenzabklingzeitbildgewinnung, PhD thesis by R. Franke. 7
8 Fluorescence (Luminescence) Life Imaging 5 HOW TO DO LUMINESCENCE LIFETIME IMAGING I 1 I 2 I 3 I 4 I 5 I 6 I 7 I 8 Figure 12: Sinusoidal luminescence signal (orange) with four sampling integration windows per period (grey rectangles), where the integrated light results in the intensity images I 1.. I 8 (I 5..I 8 are the repetition of I 1..I 4 ) In the example in figure 12 the first four integration windows have been chosen so that the intensity I 1 can be related to the sine value at Φ = 0, I 2 to Φ = 90, I 3 to Φ = 180 and finally I 4 to Φ = 270. From these four values I 1.. I 4 the phase angle Phi, the modulation index m em and the intensity of the signal can be calculated: If typical readout s for one image in modern image sensors are considered, obviously for fluorescence lifes in the range of nanoseconds four integration windows have to be recorded in a sequence of 4 images one after the other. Further, assuming a realistic modulation frequency of 20 MHz for short luminescence lifes, a proper integration window would last for a fourth of a period resulting in an exposure of 12.5 ns. This would not be enough to integrate these weak light signals. Thus, the integration is repeated many s within the overall exposure very similar to the domain measurement shown in figure 7. This would look like the integration windows shown in figure 13. Still, each single integration would be very short and difficult to achieve. For technical reasons a different evaluation or detection method based on the same assumptions and calculations can be chosen. The idea is to enlarge the integration window for the collection of more light to a half period and imitate the behavior of a two tap switch, meaning that two integrations in a sequence are performed. If we look at this situation, shown in figure 14, the integration windows cover half of a period of the sinusoidal light, and always one window is immediately followed by the other. Doing so, no photons are lost, and they all contribute to the overall acquired signal. At first, the image I 1 is recorded, which corresponds to a phase angle of Φ = 0 and immediately after a second image I 3 is recorded, which corresponds to a phase angle of Φ = 180. In a second step the synchronization between the integration windows and the detected sinusoidal luminescence signal is changed by adding an artificial phase angle of 90. Now, the integration is repeated in the same way, first the image I 2 is recorded, which corresponds to a phase angle of Φ = 90, and immediately after a second image I 4 is recorded, which corresponds to a phase angle of Φ = 270 (see fig. 14). A beneficial effect is, that the modulation signal, which controls the synchronization of the integration windows, has the same frequency as the sinusoidal luminescence signal. The next chapter will show why this method is convenient for FLIM. exposure image I 1 I 3 I 1 Φ = 0 Φ = 180 I 2 I 2 Φ = 90 I 4 Φ = 270 I 3 I 4 Figure 13: Sinusoidal luminescence signal (orange) with sampling integration windows (grey rectangles), where for each image the same part of the light waveform is collected within the same integration window. The example shows the repetitive integration at four different positions of the integration window, resulting in four images (theoretical consideration). Figure 14: Sinusoidal luminescence signal (orange) with sampling integration windows (grey rectangles). At first for the first half of the period the image I 1 is integrated, which corresponds to Φ = 0, and subsequently the image I 3 is integrated, which corresponds to Φ = 180. For the next recording the synchronization is shifted by 90, such that the first half period of integration covers I 2, which corresponds to Φ = 90, and subsequently the image I 4 is integrated, which corresponds to Φ = 270 (theoretical consideration). 8 Authors: Gerhard Holst & Robert Franke PCO AG
9 Fluorescence (Luminescence) Life Imaging 6 QMFLIM2 - A DIRECTLY MODULATABLE CMOS IMAGE SENSOR Figure 15: Top and bottom view of the package with the new QMFLIM2 CMOS image sensor the first half period and the 180 information of the modulated light signal is collected during the second half period (see figure 14). The QMFLIM2 image sensor has following general characteristics. With a resolution of 1024 x 1024 pixels equipped with microlenses it is the only directly modulatable CMOS image sensor with such a high resolution. The pixel pitch of 5.6 μm is a good fit for a combination with a microscope, and the intra-scene dynamic of 10 bit is well suited for many luminescence imaging applications. Within the research project FLI-Cam, from , a new CMOS image sensor, QMFLIM2 (see fig. 15), has been developed by the CSEM (Centre Suisse d Electronique et de Microtechnique SA, Zürich, Switzerland) and PCO. Each pixel of the image sensor has two charge collection sites, called tap A and tap B, and a switch, which can be controlled by an external signal. This configuration acts like a charge swing, which is illustrated in figure 16. The external two-level voltage signal, called modulation signal, selects whether tap A or tap B is active. If tap A is active, the photogenerated charge carriers are directed to the tap A charge bucket, when tap B is active, the carriers drift into the tap B bucket. If this switching of the signal corresponds to the zero-crossing of a sinusoidal or rectangular signal (with a duty cycle of 50%) at constant frequency, the tap A corresponds to a phase angle of 0, while tap B corresponds to a phase angle of 180. This is shown in figure 17, which is a repetition of figure 16 just with the additional information of the corresponding phase angle. Whenever the control signal for tap A (rectangular signal in figure 17 above the left bucket) is active (respectively high) the light generated charge carriers flow into the Φ = 0 (tap A) bucket, while tap B is inactive. Whenever the tap B signal is active, the charge carriers are collected in the Φ = 180 (tap B) bucket. T A B LE 1 PARAMETER resolution QMFLIM x 1024 pixels pixel size 5.6 µm x 5.6 µm frame rate modulation frequency range 90 double images/s 0-40 MHz quantum efficiency appr. 39 % dynamic range 1 : 1024 power consumption When the image sensor is read out two images are generated simultaneously, a tap A and a tap B image. Due to the image sensor architecture the light signal has to be switched off or suppressed, when the recorded double images are read out, otherwise the light falling onto the sensor will cause additional noise in the read out images. Any assymetry can be calibrated out. Alternatively the phase can alternate so that tap A recieves phase 0 half of the and phase 180 degrees for the other half. This causes the assymetry to average out but the effective frame rate is cut in half. (described in detail in chapter 7.4). 4 W This mechanism can be used to integrate over a half period of the sinusoidal signal in the given example. The 0 information of the modulated light signal is collected during 3 created after All-solid-state lock-in imaging for wide-field fluorescence life sensing, A. Esposito, 2005 Figure 16: Schematical drawing 3 of the in-pixel charge swing of the QMFLIM2 image sensor. Figure 17: Schematical drawing of the in-pixel charge swing of the QMFLIM2 image sensor with control signal and phase angle added. 9
10 Fluorescence (Luminescence) Life Imaging 7 PCO.FLIM - CAMERA SYSTEM FOR LUMINESCENCE LIFETIME IMAGING 7.1 Camera System Figure 18: Different views of the pco.flim camera system, from left to right: front view without lens side view without the water cooling connectors and back view. PARAMETER resolution pco.flim 1008 x 1008 pixels pixel size 5.6 µm x 5.6 µm T A B LE frame rate modulation frequency range (out) modulation frequency range (in) modulation signal shape 90 double images/s 5 khz - 40 MHz 500 khz - 40 MHz sinusoidal / rectangular 2 quantum efficiency typ. 39 % dynamic range 1 : 1024 A/D converter power consumption 14 bit 40 W The pco.flim camera system is the first luminescence life imaging camera using the CMOS image sensor QM- FLIM2. It offers all the required generation of frequency domain signals (5 khz 40 MHz) and also allows the use of external modulation signals in a limited frequency range (500 khz 40 MHz). It has a USB 3.0 interface for image data transfer and control of all camera operation modes. Further, a variety of trigger input / output signals for integration of the camera into any application framework is available. Table 2 gives an overview of the performance data of the camera system. 10 Authors: Gerhard Holst & Robert Franke PCO AG
11 Fluorescence (Luminescence) Life Imaging 7 PCO.FLIM - CAMERA SYSTEM FOR LUMINESCENCE LIFETIME IMAGING 7.2 Principle Structure of an Experimental Set-Up luminescent sample excitation filter modulatable light source gate signal modulation signal emission filter pco.flim imaging optics computer data interface Figure 19: Structural overview of a suggested set-up for luminescence life imaging using a pco.flim camera system. In figure 19 an overview of a set-up for luminescence life imaging using a pco.flim camera system is suggested, if the camera is the frequency master. The pco.flim camera sends the modulation signal and the dark gate signal to the light source, which must be capable of accepting both signals. While the modulation signal defines the shape of the excitation light, the gate signal controls whether the excitation light is generally switched on or off, because the light has to be switched off during the of the image readout 4. It depends on the application which modulatable light source is appropriate with respect to the required frequency range (fig. 19, modulatable light source). It can be anything from LED to laser diodes that can be properly modulated in the intended frequency range. The modulated light passes an optical excitation light filter (fig. 19, excitation filter) and will excite a luminophore in the sample of interest. It might be necessary to use additional optics to guide and shape the light to the sample. These optics are not included in the overview. The luminescent sample in turn will emit luminescence light. This light has to pass appropriate optical emission filters (fig. 19, emission filter) and will be imaged by optics (fig. 19, imaging optics) to the image sensor of the pco.flim. It is not important whether the emission has to pass first the optics and then the filter or vice versa, in figure 19 just one version is shown. The kind of optics can range from lenses to microscopes, depending on the application. According to the operation modes and settings the camera system pco.flim will transfer the images to the controlling computer (fig. 19, computer) via the USB 3.0 data interface. The components shown in figure 19 are just examples to demonstrate the flexibility of the pco.flim system. Since the camera includes the generation and control of the modulation signals, the overall set-up is relatively simple. Note: In principle the decay or life based measurements are independent of any changes in intensity. Nevertheless, in frequency domain measurements care has to be taken to efficiently block the excitation light. In frequency domain FLIM (FD-FLIM) the phase angle equivalent to the shift between excitation and emission signal is measured. Assuming that this signal is the superposition of the emission signal and for example 10% of the excitation signal (due to inefficient blocking), the phase angle of the superposed signal is measured. The phase angle of this signal depends to a large extend on the phase angle of the luminescent signal, but also on the ratio of the intensities of the unblocked excitation signal and the emission as well. Thus, any change in luminescence intensity, while the excitation usually stays constant, will introduce an additional phase angle and can cause false results. 4 The QMFLIM2 image sensor catches additional noise, when the light is on during the readout of the image sensor 11
12 Fluorescence (Luminescence) Life Imaging 7 PCO.FLIM - CAMERA SYSTEM FOR LUMINESCENCE LIFETIME IMAGING 7.3 Single Frequency Measurement I 1 Φ = 0 tap A active I 3 Φ = 180 tap B active Φ=90 I 2 Φ = 90 I 4 Φ = 270 Figure 20: Sinusoidal luminescence signal (orange) with sampling integration windows (grey rectangles). At first, for Image I 1 the first half of the period is integrated, which corresponds to tap A is active and Φ = 0, and subsequently the image I 3 is integrated, which corresponds to tap B is active and Φ = 180. For the next recording the synchronization is shifted by ΔΦ = 90, such that the first half period of integration covers I 2, which corresponds to tap A is active and Φ = 90, and subsequently the image I 4 is integrated, which corresponds to tap B is active and Φ = 270. Figure 21: Sinusoidal luminescence signal (orange) with sampling integration windows (grey rectangles). Alternately, during the first half of the period Image I 1 is integrated (tap A is active Φ = 0 ), then image I 3 is integrated during the second half (tap B is active Φ = 180 ). This is repeated many s within the frame of the exposure. For the next recording the synchronization is shifted by ΔΦ = 90, such that the first half period of integration covers I 2 (tap A is active Φ = 90 ) and subsequently the image I 4 is integrated (tap B is active Φ = 270 ). The two taps per pixel of the QMFLIM2 CMOS image sensor conducts the half period integration of a sinusoidal frequency signal as explained in chapter 5.2 and figure 14. For a better understanding this integration is combined with the charge swing schematic of the pixel in figure 20. When tap A is active it corresponds to Φ = 0 and subsequently tap B becomes active, which corresponds to Φ = 180. In both cases the sinusoidal luminescence is integrated over half a period. This procedure is repeated for a second double image, but the recording starts with 90 phase shift, such that the integration windows of tap A and tap B now correspond to the phase angles Φ of 90 and 270, respectively. In most applications, the integration of half a period of a luminescence signal will not collect enough light, therefore figure 21 shows the lapse of a real recording with four different images corresponding to Φ = , where the half period integration portions are accumulated in each pixel. Note: There is no guaranty that the overall number number of integration periods is an integer and always the same, because the exposure is independently adjusted and set from the frequency modulation. Usually, this can be neglected since the number of accumulated periods is much larger than a single period. By this method 4 images are recorded, which represent the input data for further calculations. As explained in chapter 5.2 (and in more details in the Appendix) based on these images a phase angle, a demodulation index and an intensity value can be calculated per pixel, resulting in three computed images. Figure 22: Four luminescence images corresponding to Φ = are acquired. 12 Authors: Gerhard Holst & Robert Franke PCO AG
13 Fluorescence (Luminescence) Life Imaging 7 PCO.FLIM - CAMERA SYSTEM FOR LUMINESCENCE LIFETIME IMAGING As an example from a research project four luminescence images, which have been recorded in the described manner, have been acquired (see fig. 22). Based on these four images the three images shown in figure 23 can be calculated. But at this point there are still some parameters unknown: the initial phase angle of the system including the influences of the application set-up (optics, path length of light etc.), the modulation index of the excitation light and the demodulation efficiency of the image sensor. Hence, a known (photoluminescent) reference, with similar brightness and comparable spectral behavior, has to be measured with similar settings. Subsequently the modulation index and phase angle data from figure 23 have to be referenced, which results in the false color coded distributions in figure 24. The referencing is done by subtraction of the phase angles and by division of the demodulation indices. If it is assumed that each luminescent image point can be described by a single luminescence decay or life, both images in figure 24, the modulation index distribution as well as the phase angle distribution, can be converted into corresponding life distributions, which corresponds to a different display of the same information (see fig. 25), and it represents the information which can be best interpreted by the user in most cases. Note: In most applications this assumption will be inaccurate, because it is likely that the so-called apparent life, which is calculated, is in reality the composition of different luminophores with different lifes. If several components have to be analyzed, it is necessary to repeat the measurement with different modulation frequencies and perform a proper multi-component analysis 5,6, which corresponds to the fit of multiple life decay curves in the domain. 5 Hochintegriertes Kamerasystem für die Multifrequenz-Lumineszenzabklingzeitbildgewinnung, Dissertation, Robert Franke 6 Principles of Fluorescence Spectroscopy, Joseph R. Lakowicz, Springer Verlag 7 Courtesy of Fred Wouters & Gertrude Bunt, University Medicine Göttingen Figure 23: The three result images based on the recorded images shown in figure 22. From left to right: intensity (normalized), modulation index (dimensionless), phase angle [degree] (false color coded). Courtesy of Prof. Dr. F.S. Wouters and Dr. G. Bunt, University Medicine Göttingen Figure 24: The three images out of figure 23, now referenced. From left to right: intensity (normalized), modulation index (dimensionless), phase angle [degree] (false color coded). Courtesy of Prof. Dr. F.S. Wouters and Dr. G. Bunt, University Medicine Göttingen Figure 25: The referenced modulation index and phase angle distribution images from figure 24 are converted into luminescence life distribution images. From left to right: life distributions based on modulation index and phase angle [ns] (false color coded). Courtesy of Prof. Dr. F.S. Wouters and Dr. G. Bunt, University Medicine Göttingen 13
14 Fluorescence (Luminescence) Life Imaging 7 PCO.FLIM - CAMERA SYSTEM FOR LUMINESCENCE LIFETIME IMAGING 7.3 Single Frequency Measurement Figure 26: The phasor plot of the results shown above with each pixel being represented as a vector composed of the modulation index as its absolute value and the phase angle as angle vs. the x-axis. For a convenient display, the vectors are flipped across the x-axis to place them in the upper right quadrant of the coordinate system. The color coding reflects the frequency of appearance of the corresponding vector (a histogram type coding). Very often both luminescence life distributions differ from each other, which can be a hint for the presence of multiple lifes as described in literature. Generally, the modulation index signal is more noise dependent than the phase angle signal. Another option to display the results is the use of a Nyquist plot, in FLIM applications called Phasor Plot, where both results, the modulation index and the phase angle are combined to a vector, with the absolute value given by the modulation index and the angle given by the phase angle (see fig. 26). This allows to comparison between domain and frequency domain results. The semi circle is parametrized by the fluorescence lifes and the modulation frequency. 14 Authors: Gerhard Holst & Robert Franke PCO AG
15 Fluorescence (Luminescence) Life Imaging 7 PCO.FLIM - CAMERA SYSTEM FOR LUMINESCENCE LIFETIME IMAGING 7.4 Pixel Asymmetry Figure 27: The left image shows an uncorrected image, obtained with tap A readout, while the right image shows the result image after an asymmetry correction, where the same image was read out from tap B as well, and both images have been averaged. The small images represent an extract of the area, which is denoted by the yellow square. The left image in figure 27 shows a tap A readout image of a scene outside the lab. The right image is the result of the average of a tap A and a tap B readout, which corresponds to the second optional asymmetry correction, which is described below. The small image extracts in figure 27 show a zoom of the area within the yellow square in the images for better visibility of the differences. In principle there are two options to correct for the tap asymmetry. Option 1 is an additional calibration, which should be done by the user since such a calibration would be dependent on frequency, potentially on wavelength and other parameters. Thus, it can be quite complex to foresee every possibility and store a lot of calibration data in the camera. Option 2 is the extention of the additional phase shift to cover one whole period by both taps. The pitfall of this option is the reduction of the frame rate, because it requires to record twice as much images. On the other hand there is the advantage of improving the signal-to-noise-ratio, because two images containing the same information are averaged I 1a I 3a Φ = 0 Φ = 180 tap A active Φ=90 Φ=180 Φ=270 tap B active I 2a I 4a Φ = 90 Φ = 270 tap A active I 3b Φ = 180 tap A active tap B active I 4b Φ = 270 tap A active I 1b Φ = 0 tap B active I 2b Φ = 90 tap B active Figure 28 illustrates the acquisition procedure for asymmetry correction. First, as described before, two double images I 1a.. I 4a are recorded (fig. 28, 1 and 2) with the four phase angle integration windows at Φ = Then, the recording is extended by additional two double images with an additional phase shift beyond 180 and I 1b.. I 4b are recorded (fig. 28, 3 and 4) with the same four phase angle integration windows at Φ = Comparing the sampling windows of tap A and tap B, there are four pairs of tap A and tap B containing the same integral, i.e. phase information: I 1a / I 1b, I 2a / I 2b, I 3a / I 3b, I 4a / I 4b. If now these images are averaged, the asymmetry cancels out and the signal-to-noise ratio is improved. Figure 28: Schematically, 4 double images of a sinusoidal luminescence signal (orange) are recorded with sampling integration windows (grey rectangles). 1. Images I 1a and I 3a are recorded, which correspond to Φ = 0 (tap A active) and Φ = 180 (tap B active) 2. Images I 2a and I 4a are recorded, which correspond to Φ = 90 (tap A active) and Φ = 270 (tap B active) Now, the phase is further shifted beyond 180, such that each tap sees all four phase integrals. 3. Images I 3b and I 1b are recorded, which correspond to Φ = 180 (tap A active) and Φ = 0 (tap B active) 4. Images I 4b and I 2b are recorded, which correspond to Φ = 270 (tap A active) and Φ = 90 (tap B active). 15
16 Fluorescence (Luminescence) Life Imaging 7 PCO.FLIM - CAMERA SYSTEM FOR LUMINESCENCE LIFETIME IMAGING 7.5 Fluorescent Standards from Starna Scientific To determine the initial phase of the optical system plus the camera system a reference measurement must be acquired using a fluorescence sample. This sample is excited by the same light, emits in a similar spectral range, and has a known mono-exponential fluorescence life. For this purpose we used reference materials from Starna Scientific 7, which were designed for molecular fluorescence spectrophotometry. Starna has dissolved the fluorescent materials in methylmethacrylate and the solution is then polymerized to produce a polymethylmethacrylate (PMMA) matrix, which provides a stable environment for the fluorescent compounds 8. By this means it is possible to create for example slide shaped samples, which can be placed on the microscope like the sample slides to record the reference values (see fig.29). Starna Scientific offers seven different fluorophores of which we tested and used two dyes: Compound 610 (with λ exc = 440 nm and λ em = 480 nm) which is well suited for CFP type of dyes, and Rhodamine B (with λ exc = 562 nm and λ em = 573 nm). The corrected excitation and emission spectra are shown in figures 30 and 31 (source: Starna Scientific). Starna Scientific offers to tailor the polymer material to the requested size and dimensions. We measured the following lifes of the compounds: Luminophore Measured Monoexponential Life [ns] Excitation wavelength [nm] / Emission Wavelength [nm] Compound / 490 Rhodamine B / taken from the Reference materials for Molecular Fluorescence Spectrophotometry brochure Figure 29: Sample slides with the fluorophores Compound 610 and Rhodamin B from Starna Scientific 9 two photon excitation Figure 30: Corrected excitation and emission spectra of Compound 610 Figure 31: Corrected excitation and emission spectra of Rhodamine B 16 Authors: Gerhard Holst & Robert Franke PCO AG
17 Fluorescence (Luminescence) Life Imaging 7 PCO.FLIM - CAMERA SYSTEM FOR LUMINESCENCE LIFETIME IMAGING 7.6 Proper Modulation Frequency Selection In many applications, when fluorescence lifes should be measured with a frequency domain FLIM system beside the requirement to select proper optical filters and a sufficiently fast excitation light source, the questions arises: What is an appropriate modulation frequency to measure the fluorescence life of the sample? The relationship between the phase angle Φ, the modulation frequency f mod and the fluorescence life t P was already described in chapter 5.2 and it is: If the phase angle is close to 90, a slight change in phase angle will cause huge changes in the tangent and therefore in the life value. In turn near to 0, larger changes in phase angle are required to get changes in the tangent value and therefore in the life result. If the range of lifes or the life itself is known, a good rule of thumb is to adjust the modulation frequency such that the phase angle is about 45. This means: If we have a look to the shape of the tangent function, we get the graph given in figure 32. The next table shows some examples for a variety of fluorescence and phosphorescence lifes. Luminescence Life Optimum Modulation Frequency 1 ns 159 MHz 5 ns 32 MHz 10 ns 16 MHz 50 ns 3 MHz 100 ns 1.6 MHz 500 ns 318 khz 1 µs 159 khz 10 µs 16 khz 50 µs 3.2 khz Figure 32: Graph of the tangent function 100 µs 1.6 khz The shape of the curve around an angle value of 45 is linear, which looks like a good setpoint for a frequency domain measurement. If the modulation frequency is selected such that the phase angles are either near to 0 or near to 90, the sensitivity of the measurement towards changes or differences in fluorescence lifes is not optimum. Obviously, modulation frequencies of 30 or 40 MHz are not optimum to measure lifes in the range of ns, but the autofluorescence life results (see figure 33) show that it is possible with a good sensitivity. Figure 33: Autofluorescence measurement of a lily of the valley (convallaria) slice sample, from left to right: fluorescence intensity fluorescence life distribution based on phase angle, the colorbar is given from ns fluorescence life distribution with intensity weight 17
18 Fluorescence (Luminescence) Life Imaging 7 PCO.FLIM - CAMERA SYSTEM FOR LUMINESCENCE LIFETIME IMAGING Figure 34: Fluorescence intensity image of a sample, left de-speckling switched off, right de-speckling switched on (courtesy of Rapp OptoElectronic) 7.7 Coherent Light and De-Speckling When short fluorescence lifes in the range of a couple of nanoseconds is measured the modulation frequency is near to the upper limit of the pco.flim MHz. This is often out of the useful application range of non-coherent light sources like LEDs. We tested a large number of LEDs and came to the conclusion that above 20 MHz LEDs should not be applied. Therefore it is likely that laser diodes have to be applied if the investigated fluorescence lifes are in the range of a few nanoseconds. If these coherent light sources are used, interference patterns (speckles) occur as can be seen in the left image of figure 34. If these patterns do not change during the recording of a pco.flim image stack, they have no influence on the phase angle and the modulation index distribution. required for the use of the pco.flim system needs a homogenous illumination of the viewfield of the microscope without interference patterns. Rapp OptoElectronic has developed a laser light source and a very efficient optical coupling unit (see fig. 35), which is an optimum fit for microscopy applications of the pco.flim system. The light source has all protective features, which are required for safe laser applications. It accepts the modulation and dark gate signals from the pco.flim and it is connected via an optical fiber to the combined de-speckling and optical coupling unit at the back optical port of the microscope (see fig. 35). Nevertheless with the speckle pattern in the widefield illumination, the structural interpretation of the sample image (see fig. 34 comparison between left and right image) can be quite cumbersome. The original structure of the sample (see fig. 34 right image) can be better analyzed without the speckle pattern. The company Rapp OptoElectronic has developed an excellent combination of a de-speckling unit and an optimum optical coupling unit for laser light into widefield microscopes. 7.8 Optimum Optic Coupling of Excitation Light into a Microscope Most of the available optical coupling units, which couple laser light into a microscope, usually try to illuminate spots for scanning purposes. The widefield illumination Figure 35: Microscope set-up example (Zeiss AxioObserver Z1, inverse widefield fluorescence microscope, courtesy of Zeiss AG) with Rapp Opto- Electronic laser light source and combined de-speckling and optical coupling unit, with a pco.flim camera connected to the microscope and the laser light source. 18 Authors: Gerhard Holst & Robert Franke PCO AG
19 Fluorescence (Luminescence) Life Imaging A1.1 LOCK-IN DETECTION AND CROSS-CORRELATION FUNCTION Figure A1.1: The sinusoidally modulated waveform of light incident on one pixel of the image sensor over is shown in (a). The charge carriers (electrons) are generated by absorption of light in the photodiode and directed into one of two charge buckets, called tap A and B (d). The taps are alternately selected by applying a voltage waveform shown in (b). The rectangular modulation of the tap A control voltage (blue curve) is inverted for the opposite tap B (red curve). The frequency equals that of the light modulation due to the homodyne detection. Figure (c) shows the charge carriers alternately accumulated in each tap over the entire exposure, where each tap holds the complementary information of the other tap, i.e. phase shifted by p 180. During one exposure two intensity images (all taps A and B) are created simultaneously (e). Note that in the figure only one phase relation between sensor and light modulation is shown, resulting in two phase images with a phase difference of p 180. The pco.flim s image sensor is modulated by toggling the momentarily active tap using a rectangular waveform with a duty cycle of 50%. The outgoing analog modulation signal for the excitation light has the same frequency, meaning this detection scheme is a homodyne or lock-in detection. The incident light created by the excited luminophor which is assumed to have the same number of harmonics as the outgoing analog modulation signal is mixed (i.e. multiplied) with the rectangular sensor modulation signal and integrated over in each pixel tap during the exposure. During each integration the photogenerated charge carriers are integrated in both taps per pixel, resulting in two grey values per pixel and therefore in two grey value images per integration for a given phase relation between sensor and external light modulation (see figure A1.1). Due to the symmetric toggling of both taps each tap holds the complementary information of the other tap, i.e. the information at an additional phase shift of p 180. Mathematically, this procedure is equivalent to the cross-correlation function, which is formed by phase shifting the sensor modulation signal relative to the external modulation signal. This phase shift is the independent variable of the cross-correlation function. The cross-correlation function has the property that it contains only the harmonics present in both sensor and external modulation signals. The external modulation waveform for the excitation light is often a pure sine wave which does not contain any higher harmonics. Therefore, the cross-correlation function is a sine wave as well. Due to the fact that the light intensity cannot become negative the cross-correlation function carries a constant component as well as the incident light does. To reconstruct the sinusoidally shaped cross-correlation function it has to be sampled at three or more sampling points due to the Nyquist criterion. For that purpose at least three integrations at different phases have to be performed. The pco.flim camera supports powers of two as a number of equidistant phases, where 4, 8 or 16 phases are possible. For special applications (e.g. ratio imaging) a phase number of two is also available, simply halving the modulation period. There are two possibilities to acquire the intensity images to cover all intended phases per modulation period. The first way is to use the first tap (called tap A ) to cover the first half period, whereas the second tap (called tap B ) automatically covers the second half period as it holds the complementary information as described above. Assuming a certain asymmetry between both taps A and B, which can actually be observed using the pco.flim, this method can lead to slight distortions of the computed results. To overcome this problem, the second method is to shift the phase throughout the complete modulation period, so that each tap covers all phase steps. By applying a subsequent averaging of taps A and B holding the same phase information the asymmetry can be compensated. A drawback of this method is the necessity of acquiring twice as many images resulting in half the effective frame rate of the computed results. Note: Even if sinusoidally modulated light with the maximum modulation index of 1 (i.e. showing a possible momentary value of zero) is incident on the image sensor, the demodulation index of the cross-correlation (sine) function is theoretically 2 p 64%, since each tap integrates over a half period of a sine wave with positive values only and cannot become zero. This reduction factor is automatically compensated during referencing (see Referencing). 19
20 Fluorescence (Luminescence) Life Imaging A1.2 NUMERICAL HARMONIC ANALYSIS If a pure sinusoidal excitation light waveform is used, the cross-correlation function mentioned above has a sinusoidal waveform as well, assuming a linear -invariant photoluminescent sample. Therefore the following formulae refer to the analysis of the first harmonic, i.e. the fundamental component, and the discrete complex Fourier transform is simplified. The modulation period 2p 360 is divided into N equidistant phases indexed by k = 0 N 1. For the pco.flim camera valid values are N {2, 4, 8, 16}. For each phase step k the intensity (grey value) of each pixel (x, y) is given by Ixy [k]. The demodulation amplitude for each pixel (x, y) of the image is given by a xy = 2 N 1 I xy [k]e i 2π N k = 2 N 1 I xy [k] w k N N k=0 k=0 The constant component, i.e. mean value, is given by b xy = 1 N 1 I xy [k] N k=0 To obtain the demodulation index the amplitude is normalized by the constant component m xy = a xy b xy The computed two-dimensional arrays m and f, containing real values only, can be displayed as images of the same size as the grey value raw images using an appropriate intensity and/or color coding. The constant component image b can be displayed as a normal grey value image. Both real parameters, the demodulation index and the phase, can also be combined into a complex parameter in polar form, the so-called phasor and the phase is given by φ xy = arg ( N 1 ) I xy [k]e i 2π N k = arg k=0 ( N 1 ) I xy [k] w k k=0 with the imaginary unit i = 1 and the roots of unity which can also be written using Euler s formula to split the complex relation into two real equations, i.e. for the real and imaginary part ( ) ( ) w k =e i 2π 2π 2π N k = cos N k i sin N k For a given N the roots of unity can be computed in advance for k = 0 N 1 and stored in an array: w k = w [k] Then, the demodulation amplitude and phase can be computed by the sum of products: a xy = 2 N 1 I xy [k] w [k] N φ xy = arg k=0 ( N 1 ) I xy [k] w [k] k=0 Instead of displaying the computed images m and f all complex phasors, whose quantity equals the number of pixels in the image, can be displayed by marking their complex value within the complex plane. Such a plot is called Nyquist plot or phasor plot. A color coding can be used to indicate the frequency of appearance within a certain proximity in the complex plane, creating a kind of density clouds. Example p xy = m xy e iφxy The following example demonstrates the computation of the parameters mentioned above for four phases per modulation period, i.e. N = 4. The array containing the roots of unity is initialized with w [0] = 1, w[1] = i, w [2] = 1, w[3] = i The demodulation amplitude is computed by means of the sum of the intensity values weighted with the corresponding roots of unity a xy = 2 N I xy [0] ii xy [1] I xy [2] + ii xy [3] = 2 N I xy [0] I xy [2] + i (I xy [3] I xy [1]) = 1 (I xy [0] I xy [2]) 2 +(I xy [3] I xy [1]) Authors: Gerhard Holst & Robert Franke PCO AG
21 Fluorescence (Luminescence) Life Imaging A1.2 NUMERICAL HARMONIC ANALYSIS For simpler computation the real part is separated from the imaginary part to apply the Pythagorean theorem to obtain the magnitude of the complex sum. This separation can also be used to express tan fxy as the quotient of imaginary part divided by the real part, giving the following equation for the phase φ xy = arctan ( ) Ixy [3] I xy [1] I xy [0] I xy [2] ) ( ) Ixy [1] I xy [3] = arctan I xy [0] I xy [2] If the real part becomes negative the corresponding phase must be located in the second or third quadrant, depending on the sign of the imaginary part. In that case the adjustment can simply be done by adding p to the phase fxy computed in the above formula. The constant component is computed by b xy = 1 4 (I xy [0] + I xy [1] + I xy [2] + I xy [3]) To be more precise, the signs of the real and imaginary part have to be examined to adjust the computed phase fxy to lie within all four possible quadrants of the complex plane since the principle values of the arctan function only cover the first and fourth quadrant, i.e. p / 2 fxy p / 2. giving the demodulation index m xy = 2 (I xy [0] I xy [2]) 2 +(I xy [3] I xy [1]) 2 I xy [0] + I xy [1] + I xy [2] + I xy [3] A1.3 ORDER OF PHASE IMAGES USING THE PCO.FLIM CAMERA The intensity images used in the computations mentioned above are supposed to be in an ascending order with respect to their phases as the roots of unity are indexed in an ascending order as well. However, the pco.flim camera delivers the intensity images in different possible phase orders, since both taps A and B, if both selected, are read out alternately. Due to various possible camera settings there are different resulting phase ordering schemes, which have to be considered in order to apply the above formulae correctly. Because tap B automatically carries the phase information shifted by p 180 relative to tap A, it can be sufficient to choose a phase shifting mode, which lets tap A cover the first half period of one modulation period and tap B the second half. Each phase of one full modulation period is covered by one single tap only, calling this setting singular. As mentioned in section Lock-In Detection and Cross-Correlation Function a certain asymmetry between both taps can it make necessary to cover each phase information by both taps, which is done using the setting twice. Therefore, the resulting phase sequences are twice as long as in singular mode. To ease the application of different calculations on the image sequence, the order of the different phase shifts can be altered. Using the ascending mode the phase is linearly shifted by one phase increment after both taps A and B have been integrated and read out, scanning the modulation period in an ascending manner. In order to easily apply an external or internal asymmetry correction by averaging two corresponding taps, the sequence order can be set to opposite. This mode will re-sort the image sequence in a way that two sequent pairs (taps A and B) carry information of two phases with inverted phases in the second pair, e.g. 0 (tap A), 180 (tap B), 180 (tap A), 0 (tap B), etc. Now, the averaging of taps A and B can be applied on the phase information 0, 180, etc. Note that both modes ascending and opposite have only effect if the first mode twice has been selected. While both taps A and B are always integrated and read out of the image sensor, the user can select, whether tap A, tap B or both (alternately) are output at the camera interface via the options tap A, tap B or both. The following examples show several combinations of the parameters mentioned above resulting in different image sequences. The parameter designations are those used in the SDK description (see pco.flim SDK Manual for more information). All image sequences are re-sorted into a uniform ascending sequence of phase images for the further computation of phase, demodulation index and mean intensity images. Example 1: Parameter Phase Number Phase Symmetry Phase Order Tap Select Asymmetry Correction Value [4 phases] [singular] [ascending] [both] [off] 21
22 Fluorescence (Luminescence) Life Imaging A1.3 ORDER OF PHASE IMAGES USING THE PCO.FLIM CAMERA Four phases per modulation period are selected, where each phase is covered by only one tap ( singular mode). The phase order is set to ascending, putting each tap into an ascending order of phase increments, i.e. 0 and 90 for tap A and 180 and 270 for tap B. Due to the sensor readout scheme taps A and B are always output alternately, if both are selected. re-sorting the original image sequence with four phase images is obtained. In the above figure the top row shows the sequence order of phase images out of the camera. The phase images have to be (virtually) re-sorted to an ascending phase sequence for the computation of the phase, demodulation index and intensity images (bottom row). Example 2: Parameter Phase Number Phase Symmetry Phase Order The mode phase symmetry is now set to twice, where each phase is covered twice by tap A and B, resulting in a sequence length of eight in case that both taps would be selected. In the first step, shown in this example, only tap A is picked, resulting in a sequence containing four tap A phase images in an ascending phase order. Example 3: Value [4 phases] [twice] [ascending] Tap Select [tap A] Asymmetry Correction Parameter Phase Number Phase Symmetry Phase Order Tap Select Asymmetry Correction [off] Value [4 phases] [twice] [ascending] [both] [off] By selecting both taps in the example above, the resulting sequence length is eight. Each tap steps through the full modulation period in an ascending order, while taps A and B are output alternately as always. By externally averaging taps A and B containing the same phase information and Example 4: Parameter Phase Number Phase Symmetry Phase Order Tap Select Asymmetry Correction The phase order of the example above is now changed to opposite instead of ascending, creating two sequential pairs of taps A and B with swapped phase information. The same averaging principle as mentioned above is externally applied. Example 5: Parameter Phase Number Phase Symmetry Phase Order Tap Select Asymmetry Correction Value [4 phases] [twice] [opposite] [both] [off] Value [4 phases] [twice] [opposite] [both] [average] The settings of the last example are required to meaningfully use the internal averaging mode by changing the parameter asymmetry correction to average. Eight phase images per sequence are internally recorded and averaged in the same way as externally performed in the above examples. The resulting sequence length is four with the same phase sorting as in the first example. 22 Authors: Gerhard Holst & Robert Franke PCO AG
23 Fluorescence (Luminescence) Life Imaging 5 HOW TO DO LUMINESCENCE LIFETIME IMAGING A1.4 REFERENCING A complete measuring setup, including the camera (image sensor, analog modulation circuitry), modulated light source and optical path length, exhibits initial values of the overall modulation index and phase, independent of the photoluminescent sample itself, which usually differ from the ideal initial values: a modulation depth of one (a theoretical demodulation depth of 2 p 64%, see Lock-In Detection and Cross-Correlation Function) and a phase shift of zero (neglecting the delay induced by the optical path length and the finite velocity of light). To correct the deviations from the ideal values, a reference measurement has to be performed by replacing the sample of interest by a reference sample with known characteristics, i.e. with a known luminescent life. By means of referencing, the influences of the whole measuring setup are compensated, since they affect the reference and sample measurements the same way. The recommended reference method uses a reference luminophore with a mono-exponential behavior, a known luminescent life t ref and a luminescence emission in a similar spectral range as the sample of interest. The referenced phasor for a given modulation frequency f is then computed by p = 1 1+iωτ ref pem p ref with the circular frequency w = 2pf. The first right-hand complex term describes a first-order low-pass system introducing an additional phase shift and decrease of the modulation index. This term is valid and constant for all pixels of the image, since it describes one single underlying model. The equation can be rewritten in Euler s form The simplest reference would be a reflecting or scattering target which does not alter the modulation index and phase by intrinsic properties like a luminophore does. It would have a constant or life of zero. To calculate the referenced phasor p of the sample of interest, the phasor obtained by the sample measurement p em is normalized by the phasor obtained by the scattered or reflected excitation p exc by division of both phasors: = e i arctan(ωτ ref) p = mem e i(φem φ ref ) = 1+ω2 τref 2 m ref m em m ref 1+ω2 τ 2 ref e i(φem φ ref arctan(ωτ ref )) p = p em p exc = m eme iφem m exc e iφexc = m em m exc e i(φem φ exc) = me iφ The demodulation indices are divided by each other and the phases are subtracted. This method is based on the assumption that the measuring system exhibits the same influences on the sample and reference (excitation) measurements, which is not true for most applications. The modulated image sensor shows wavelength dependent demodulation properties which are relevant, since excitation and luminescence usually are spectrally different. with the referenced modulation index m em m = m ref 1+ω2 τref 2 and the referenced phase φ = φ em φ ref arctan (ωτ ref ) 23
24 Fluorescence (Luminescence) Life Imaging A1.5 SAMPLING THEORY - PCO.FLIM RESULT IMAGES Theory Sampling Theory pco.flim Operation pco.flim Recorded Images pco.flim 1. 1 phase image pair: Φ = exposure per double image 2. 2 phase image pairs: Φ = Fluorescence Emission Signal / Light 4 phase image pairs: Φ = Authors: Gerhard Holst & Robert Franke PCO AG
25 find us europe PCO AG Donaupark Kelheim, Germany fon +49 (0) fax +49 (0) america PCO-TECH Inc Metroplex Drive Romulus, Michigan 48174, USA fon +1 (248) fax +1 (248) asia PCO Imaging Asia Pte. 3 Temasek Ave Centennial Tower, Level 34 Singapore, fon fax info@pco-imaging.de subject to changes without prior notice I PCO AG, Kelheim pco.flim whitepaper v1.01
Fluorescence (Luminescence) Lifetime Imaging application simplified... with the pco.flim
 Fluorescence (Luminescence) Lifetime Imaging application simplified... with the pco.flim lifetimes from 100 ps to 100 µs unique resolution 1008 x 1008 pixels high frame rate up to 90 fps frequency synthesizer
Fluorescence (Luminescence) Lifetime Imaging application simplified... with the pco.flim lifetimes from 100 ps to 100 µs unique resolution 1008 x 1008 pixels high frame rate up to 90 fps frequency synthesizer
PCS-150 / PCI-200 High Speed Boxcar Modules
 Becker & Hickl GmbH Kolonnenstr. 29 10829 Berlin Tel. 030 / 787 56 32 Fax. 030 / 787 57 34 email: info@becker-hickl.de http://www.becker-hickl.de PCSAPP.DOC PCS-150 / PCI-200 High Speed Boxcar Modules
Becker & Hickl GmbH Kolonnenstr. 29 10829 Berlin Tel. 030 / 787 56 32 Fax. 030 / 787 57 34 email: info@becker-hickl.de http://www.becker-hickl.de PCSAPP.DOC PCS-150 / PCI-200 High Speed Boxcar Modules
Practical work no. 3: Confocal Live Cell Microscopy
 Practical work no. 3: Confocal Live Cell Microscopy Course Instructor: Mikko Liljeström (MIU) 1 Background Confocal microscopy: The main idea behind confocality is that it suppresses the signal outside
Practical work no. 3: Confocal Live Cell Microscopy Course Instructor: Mikko Liljeström (MIU) 1 Background Confocal microscopy: The main idea behind confocality is that it suppresses the signal outside
TCSPC at Wavelengths from 900 nm to 1700 nm
 TCSPC at Wavelengths from 900 nm to 1700 nm We describe picosecond time-resolved optical signal recording in the spectral range from 900 nm to 1700 nm. The system consists of an id Quantique id220 InGaAs
TCSPC at Wavelengths from 900 nm to 1700 nm We describe picosecond time-resolved optical signal recording in the spectral range from 900 nm to 1700 nm. The system consists of an id Quantique id220 InGaAs
Megapixel FLIM with bh TCSPC Modules
 Megapixel FLIM with bh TCSPC Modules The New SPCM 64-bit Software Abstract: Becker & Hickl have recently introduced version 9.60 of their SPCM TCSPC data acquisition software. SPCM version 9.60 not only
Megapixel FLIM with bh TCSPC Modules The New SPCM 64-bit Software Abstract: Becker & Hickl have recently introduced version 9.60 of their SPCM TCSPC data acquisition software. SPCM version 9.60 not only
TCSPC measurements with the InGaAs/InP Single- photon counter
 TCSPC measurements with the InGaAs/InP Single-photon counter A typical setup in which the InGaAs/InP Single- Photon Detection Module is widely employed is a photon- timing one, as illustrated in Figure
TCSPC measurements with the InGaAs/InP Single-photon counter A typical setup in which the InGaAs/InP Single- Photon Detection Module is widely employed is a photon- timing one, as illustrated in Figure
Detectors for microscopy - CCDs, APDs and PMTs. Antonia Göhler. Nov 2014
 Detectors for microscopy - CCDs, APDs and PMTs Antonia Göhler Nov 2014 Detectors/Sensors in general are devices that detect events or changes in quantities (intensities) and provide a corresponding output,
Detectors for microscopy - CCDs, APDs and PMTs Antonia Göhler Nov 2014 Detectors/Sensors in general are devices that detect events or changes in quantities (intensities) and provide a corresponding output,
DCS-120. Confocal Scanning FLIM Systems. Based on bh s Multidimensional Megapixel FLIM Technology
 Based on bh s Multidimensional Megapixel FLIM Technology Complete Laser Scanning FLIM Microscopes FLIM Upgrades for Existing Conventional Microscopes Multidimensional TCSPC technique High throughput dual-channel
Based on bh s Multidimensional Megapixel FLIM Technology Complete Laser Scanning FLIM Microscopes FLIM Upgrades for Existing Conventional Microscopes Multidimensional TCSPC technique High throughput dual-channel
Digital Camera Technologies for Scientific Bio-Imaging. Part 2: Sampling and Signal
 Digital Camera Technologies for Scientific Bio-Imaging. Part 2: Sampling and Signal Yashvinder Sabharwal, 1 James Joubert 2 and Deepak Sharma 2 1. Solexis Advisors LLC, Austin, TX, USA 2. Photometrics
Digital Camera Technologies for Scientific Bio-Imaging. Part 2: Sampling and Signal Yashvinder Sabharwal, 1 James Joubert 2 and Deepak Sharma 2 1. Solexis Advisors LLC, Austin, TX, USA 2. Photometrics
3D light microscopy techniques
 3D light microscopy techniques The image of a point is a 3D feature In-focus image Out-of-focus image The image of a point is not a point Point Spread Function (PSF) 1D imaging 2D imaging 3D imaging Resolution
3D light microscopy techniques The image of a point is a 3D feature In-focus image Out-of-focus image The image of a point is not a point Point Spread Function (PSF) 1D imaging 2D imaging 3D imaging Resolution
An 8-Channel Parallel Multispectral TCSPC FLIM System
 An 8-Channel Parallel Multispectral TCSPC FLIM System Abstract. We describe a TCSPC FLIM system that uses 8 parallel TCSPC channels to record FLIM data at a peak count rate on the order of 50 10 6 s -1.
An 8-Channel Parallel Multispectral TCSPC FLIM System Abstract. We describe a TCSPC FLIM system that uses 8 parallel TCSPC channels to record FLIM data at a peak count rate on the order of 50 10 6 s -1.
Spectroscopy of Ruby Fluorescence Physics Advanced Physics Lab - Summer 2018 Don Heiman, Northeastern University, 1/12/2018
 1 Spectroscopy of Ruby Fluorescence Physics 3600 - Advanced Physics Lab - Summer 2018 Don Heiman, Northeastern University, 1/12/2018 I. INTRODUCTION The laser was invented in May 1960 by Theodor Maiman.
1 Spectroscopy of Ruby Fluorescence Physics 3600 - Advanced Physics Lab - Summer 2018 Don Heiman, Northeastern University, 1/12/2018 I. INTRODUCTION The laser was invented in May 1960 by Theodor Maiman.
product overview pco.edge family the most versatile scmos camera portfolio on the market pioneer in scmos image sensor technology
 product overview family the most versatile scmos camera portfolio on the market pioneer in scmos image sensor technology scmos knowledge base scmos General Information PCO scmos cameras are a breakthrough
product overview family the most versatile scmos camera portfolio on the market pioneer in scmos image sensor technology scmos knowledge base scmos General Information PCO scmos cameras are a breakthrough
PERFORMANCE OF PHOTODIGM S DBR SEMICONDUCTOR LASERS FOR PICOSECOND AND NANOSECOND PULSING APPLICATIONS
 PERFORMANCE OF PHOTODIGM S DBR SEMICONDUCTOR LASERS FOR PICOSECOND AND NANOSECOND PULSING APPLICATIONS By Jason O Daniel, Ph.D. TABLE OF CONTENTS 1. Introduction...1 2. Pulse Measurements for Pulse Widths
PERFORMANCE OF PHOTODIGM S DBR SEMICONDUCTOR LASERS FOR PICOSECOND AND NANOSECOND PULSING APPLICATIONS By Jason O Daniel, Ph.D. TABLE OF CONTENTS 1. Introduction...1 2. Pulse Measurements for Pulse Widths
IR Antibunching Measurements with id201 InGaAs Gated SPAD Detectors
 IR Antibunching Measurements with id201 GaAs Gated SPAD Detectors Abstract. Antibunching measurements with GaAs SPAD detectors are faced with the problems of high background count rate, afterpulsing, and
IR Antibunching Measurements with id201 GaAs Gated SPAD Detectors Abstract. Antibunching measurements with GaAs SPAD detectors are faced with the problems of high background count rate, afterpulsing, and
3D light microscopy techniques
 3D light microscopy techniques The image of a point is a 3D feature In-focus image Out-of-focus image The image of a point is not a point Point Spread Function (PSF) 1D imaging 1 1 2! NA = 0.5! NA 2D imaging
3D light microscopy techniques The image of a point is a 3D feature In-focus image Out-of-focus image The image of a point is not a point Point Spread Function (PSF) 1D imaging 1 1 2! NA = 0.5! NA 2D imaging
Photons and solid state detection
 Photons and solid state detection Photons represent discrete packets ( quanta ) of optical energy Energy is hc/! (h: Planck s constant, c: speed of light,! : wavelength) For solid state detection, photons
Photons and solid state detection Photons represent discrete packets ( quanta ) of optical energy Energy is hc/! (h: Planck s constant, c: speed of light,! : wavelength) For solid state detection, photons
Technical Notes. Integrating Sphere Measurement Part II: Calibration. Introduction. Calibration
 Technical Notes Integrating Sphere Measurement Part II: Calibration This Technical Note is Part II in a three part series examining the proper maintenance and use of integrating sphere light measurement
Technical Notes Integrating Sphere Measurement Part II: Calibration This Technical Note is Part II in a three part series examining the proper maintenance and use of integrating sphere light measurement
A Short History of Using Cameras for Weld Monitoring
 A Short History of Using Cameras for Weld Monitoring 2 Background Ever since the development of automated welding, operators have needed to be able to monitor the process to ensure that all parameters
A Short History of Using Cameras for Weld Monitoring 2 Background Ever since the development of automated welding, operators have needed to be able to monitor the process to ensure that all parameters
DCS-120. Confocal Scanning FLIM Systems. Based on bh s Multidimensional Megapixel FLIM Technology
 DCS-120 Based on bh s Multidimensional Megapixel FLIM Technology Complete Laser Scanning FLIM Microscopes FLIM Upgrades for Existing Conventional Microscopes FLIM with up to 2048 x 2048 pixels Decay curves
DCS-120 Based on bh s Multidimensional Megapixel FLIM Technology Complete Laser Scanning FLIM Microscopes FLIM Upgrades for Existing Conventional Microscopes FLIM with up to 2048 x 2048 pixels Decay curves
Luminescent Background Sources and Corrections
 Concept Tech Note 1 Luminescent Background Sources and Corrections The background sources of light from luminescent images are inherently very low. This appendix discusses sources of background and how
Concept Tech Note 1 Luminescent Background Sources and Corrections The background sources of light from luminescent images are inherently very low. This appendix discusses sources of background and how
Low Cost Earth Sensor based on Oxygen Airglow
 Assessment Executive Summary Date : 16.06.2008 Page: 1 of 7 Low Cost Earth Sensor based on Oxygen Airglow Executive Summary Prepared by: H. Shea EPFL LMTS herbert.shea@epfl.ch EPFL Lausanne Switzerland
Assessment Executive Summary Date : 16.06.2008 Page: 1 of 7 Low Cost Earth Sensor based on Oxygen Airglow Executive Summary Prepared by: H. Shea EPFL LMTS herbert.shea@epfl.ch EPFL Lausanne Switzerland
Supplemental Information
 Optically Activated Delayed Fluorescence Blake C. Fleischer, Jeffrey T. Petty, Jung-Cheng Hsiang, Robert M. Dickson, * School of Chemistry & Biochemistry and Petit Institute for Bioengineering and Bioscience,
Optically Activated Delayed Fluorescence Blake C. Fleischer, Jeffrey T. Petty, Jung-Cheng Hsiang, Robert M. Dickson, * School of Chemistry & Biochemistry and Petit Institute for Bioengineering and Bioscience,
Redefining Measurement ID101 OEM Visible Photon Counter
 Redefining Measurement ID OEM Visible Photon Counter Miniature Photon Counter for OEM Applications Intended for large-volume OEM applications, the ID is the smallest, most reliable and most efficient single-photon
Redefining Measurement ID OEM Visible Photon Counter Miniature Photon Counter for OEM Applications Intended for large-volume OEM applications, the ID is the smallest, most reliable and most efficient single-photon
Boulevard du Temple Daguerrotype (Paris,1838) a busy street? Nyquist sampling for movement
 Boulevard du Temple Daguerrotype (Paris,1838) a busy street? Nyquist sampling for movement CONFOCAL MICROSCOPY BioVis Uppsala, 2017 Jeremy Adler Matyas Molnar Dirk Pacholsky Widefield & Confocal Microscopy
Boulevard du Temple Daguerrotype (Paris,1838) a busy street? Nyquist sampling for movement CONFOCAL MICROSCOPY BioVis Uppsala, 2017 Jeremy Adler Matyas Molnar Dirk Pacholsky Widefield & Confocal Microscopy
Lecture 18: Photodetectors
 Lecture 18: Photodetectors Contents 1 Introduction 1 2 Photodetector principle 2 3 Photoconductor 4 4 Photodiodes 6 4.1 Heterojunction photodiode.................... 8 4.2 Metal-semiconductor photodiode................
Lecture 18: Photodetectors Contents 1 Introduction 1 2 Photodetector principle 2 3 Photoconductor 4 4 Photodiodes 6 4.1 Heterojunction photodiode.................... 8 4.2 Metal-semiconductor photodiode................
Single-photon excitation of morphology dependent resonance
 Single-photon excitation of morphology dependent resonance 3.1 Introduction The examination of morphology dependent resonance (MDR) has been of considerable importance to many fields in optical science.
Single-photon excitation of morphology dependent resonance 3.1 Introduction The examination of morphology dependent resonance (MDR) has been of considerable importance to many fields in optical science.
Camera Test Protocol. Introduction TABLE OF CONTENTS. Camera Test Protocol Technical Note Technical Note
 Technical Note CMOS, EMCCD AND CCD CAMERAS FOR LIFE SCIENCES Camera Test Protocol Introduction The detector is one of the most important components of any microscope system. Accurate detector readings
Technical Note CMOS, EMCCD AND CCD CAMERAS FOR LIFE SCIENCES Camera Test Protocol Introduction The detector is one of the most important components of any microscope system. Accurate detector readings
Instructions for the Experiment
 Instructions for the Experiment Excitonic States in Atomically Thin Semiconductors 1. Introduction Alongside with electrical measurements, optical measurements are an indispensable tool for the study of
Instructions for the Experiment Excitonic States in Atomically Thin Semiconductors 1. Introduction Alongside with electrical measurements, optical measurements are an indispensable tool for the study of
The new CMOS Tracking Camera used at the Zimmerwald Observatory
 13-0421 The new CMOS Tracking Camera used at the Zimmerwald Observatory M. Ploner, P. Lauber, M. Prohaska, P. Schlatter, J. Utzinger, T. Schildknecht, A. Jaeggi Astronomical Institute, University of Bern,
13-0421 The new CMOS Tracking Camera used at the Zimmerwald Observatory M. Ploner, P. Lauber, M. Prohaska, P. Schlatter, J. Utzinger, T. Schildknecht, A. Jaeggi Astronomical Institute, University of Bern,
III III 0 IIOI DID IIO 1101 I II 0II II 100 III IID II DI II
 (19) United States III III 0 IIOI DID IIO 1101 I0 1101 0II 0II II 100 III IID II DI II US 200902 19549A1 (12) Patent Application Publication (10) Pub. No.: US 2009/0219549 Al Nishizaka et al. (43) Pub.
(19) United States III III 0 IIOI DID IIO 1101 I0 1101 0II 0II II 100 III IID II DI II US 200902 19549A1 (12) Patent Application Publication (10) Pub. No.: US 2009/0219549 Al Nishizaka et al. (43) Pub.
Nikon. King s College London. Imaging Centre. N-SIM guide NIKON IMAGING KING S COLLEGE LONDON
 N-SIM guide NIKON IMAGING CENTRE @ KING S COLLEGE LONDON Starting-up / Shut-down The NSIM hardware is calibrated after system warm-up occurs. It is recommended that you turn-on the system for at least
N-SIM guide NIKON IMAGING CENTRE @ KING S COLLEGE LONDON Starting-up / Shut-down The NSIM hardware is calibrated after system warm-up occurs. It is recommended that you turn-on the system for at least
PZ-FLIM-110. Piezo Scanning FLIM System. Based on bh s Megapixel FLIM Technology. Complete FLIM Microscopes FLIM Upgrades for Existing Microscopes
 Based on bh s Megapixel FLIM Technology Complete FLIM Microscopes FLIM Upgrades for Existing Microscopes Multidimensional TCSPC technique Sample Scanning by Piezo Stage Compact Electronics, Controlled
Based on bh s Megapixel FLIM Technology Complete FLIM Microscopes FLIM Upgrades for Existing Microscopes Multidimensional TCSPC technique Sample Scanning by Piezo Stage Compact Electronics, Controlled
2013 LMIC Imaging Workshop. Sidney L. Shaw Technical Director. - Light and the Image - Detectors - Signal and Noise
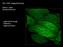 2013 LMIC Imaging Workshop Sidney L. Shaw Technical Director - Light and the Image - Detectors - Signal and Noise The Anatomy of a Digital Image Representative Intensities Specimen: (molecular distribution)
2013 LMIC Imaging Workshop Sidney L. Shaw Technical Director - Light and the Image - Detectors - Signal and Noise The Anatomy of a Digital Image Representative Intensities Specimen: (molecular distribution)
Wide-Field TCSPC FLIM with bh SPC-150 N TCSPC System and Photek FGN Detector
 Wide-Field TCSPC FLIM with bh SPC-150 N TCSPC System and Photek FGN 392-1000 Detector Abstract: We present a wide-field TCSPC FLIM system consisting of a position-sensitive MCP PMT of the delay-line type,
Wide-Field TCSPC FLIM with bh SPC-150 N TCSPC System and Photek FGN 392-1000 Detector Abstract: We present a wide-field TCSPC FLIM system consisting of a position-sensitive MCP PMT of the delay-line type,
Akinori Mitani and Geoff Weiner BGGN 266 Spring 2013 Non-linear optics final report. Introduction and Background
 Akinori Mitani and Geoff Weiner BGGN 266 Spring 2013 Non-linear optics final report Introduction and Background Two-photon microscopy is a type of fluorescence microscopy using two-photon excitation. It
Akinori Mitani and Geoff Weiner BGGN 266 Spring 2013 Non-linear optics final report Introduction and Background Two-photon microscopy is a type of fluorescence microscopy using two-photon excitation. It
Applications of Steady-state Multichannel Spectroscopy in the Visible and NIR Spectral Region
 Feature Article JY Division I nformation Optical Spectroscopy Applications of Steady-state Multichannel Spectroscopy in the Visible and NIR Spectral Region Raymond Pini, Salvatore Atzeni Abstract Multichannel
Feature Article JY Division I nformation Optical Spectroscopy Applications of Steady-state Multichannel Spectroscopy in the Visible and NIR Spectral Region Raymond Pini, Salvatore Atzeni Abstract Multichannel
Maria Smedh, Centre for Cellular Imaging. Maria Smedh, Centre for Cellular Imaging
 Nonlinear microscopy I: Two-photon fluorescence microscopy Multiphoton Microscopy What is multiphoton imaging? Applications Different imaging modes Advantages/disadvantages Scattering of light in thick
Nonlinear microscopy I: Two-photon fluorescence microscopy Multiphoton Microscopy What is multiphoton imaging? Applications Different imaging modes Advantages/disadvantages Scattering of light in thick
Shreyash Tandon M.S. III Year
 Shreyash Tandon M.S. III Year 20091015 Confocal microscopy is a powerful tool for generating high-resolution images and 3-D reconstructions of a specimen by using point illumination and a spatial pinhole
Shreyash Tandon M.S. III Year 20091015 Confocal microscopy is a powerful tool for generating high-resolution images and 3-D reconstructions of a specimen by using point illumination and a spatial pinhole
Chapter 3 OPTICAL SOURCES AND DETECTORS
 Chapter 3 OPTICAL SOURCES AND DETECTORS 3. Optical sources and Detectors 3.1 Introduction: The success of light wave communications and optical fiber sensors is due to the result of two technological breakthroughs.
Chapter 3 OPTICAL SOURCES AND DETECTORS 3. Optical sources and Detectors 3.1 Introduction: The success of light wave communications and optical fiber sensors is due to the result of two technological breakthroughs.
Nikon Instruments Europe
 Nikon Instruments Europe Recommendations for N-SIM sample preparation and image reconstruction Dear customer, We hope you find the following guidelines useful in order to get the best performance out of
Nikon Instruments Europe Recommendations for N-SIM sample preparation and image reconstruction Dear customer, We hope you find the following guidelines useful in order to get the best performance out of
DETECTORS Important characteristics: 1) Wavelength response 2) Quantum response how light is detected 3) Sensitivity 4) Frequency of response
 DETECTORS Important characteristics: 1) Wavelength response 2) Quantum response how light is detected 3) Sensitivity 4) Frequency of response (response time) 5) Stability 6) Cost 7) convenience Photoelectric
DETECTORS Important characteristics: 1) Wavelength response 2) Quantum response how light is detected 3) Sensitivity 4) Frequency of response (response time) 5) Stability 6) Cost 7) convenience Photoelectric
FLIM on a wide field fluorescence microscope
 FLIM on a wide field fluorescence microscope L.K. van Geest, K.W.J. Stoop Lambert Instruments, Turfweg 4, 933TH Leutingewolde, The Netherlands. Phone: +3 50 50846, Fax: +3 50 500034, e-mail: lkvgeest@lambert-instruments.com
FLIM on a wide field fluorescence microscope L.K. van Geest, K.W.J. Stoop Lambert Instruments, Turfweg 4, 933TH Leutingewolde, The Netherlands. Phone: +3 50 50846, Fax: +3 50 500034, e-mail: lkvgeest@lambert-instruments.com
Multifluorescence The Crosstalk Problem and Its Solution
 Multifluorescence The Crosstalk Problem and Its Solution If a specimen is labeled with more than one fluorochrome, each image channel should only show the emission signal of one of them. If, in a specimen
Multifluorescence The Crosstalk Problem and Its Solution If a specimen is labeled with more than one fluorochrome, each image channel should only show the emission signal of one of them. If, in a specimen
pco.edge 4.2 LT 0.8 electrons 2048 x 2048 pixel 40 fps up to :1 up to 82 % pco. low noise high resolution high speed high dynamic range
 edge 4.2 LT scientific CMOS camera high resolution 2048 x 2048 pixel low noise 0.8 electrons USB 3.0 small form factor high dynamic range up to 37 500:1 high speed 40 fps high quantum efficiency up to
edge 4.2 LT scientific CMOS camera high resolution 2048 x 2048 pixel low noise 0.8 electrons USB 3.0 small form factor high dynamic range up to 37 500:1 high speed 40 fps high quantum efficiency up to
Light, Color, Spectra 05/30/2006. Lecture 17 1
 What do we see? Light Our eyes can t t detect intrinsic light from objects (mostly infrared), unless they get red hot The light we see is from the sun or from artificial light When we see objects, we see
What do we see? Light Our eyes can t t detect intrinsic light from objects (mostly infrared), unless they get red hot The light we see is from the sun or from artificial light When we see objects, we see
Microscopic Structures
 Microscopic Structures Image Analysis Metal, 3D Image (Red-Green) The microscopic methods range from dark field / bright field microscopy through polarisation- and inverse microscopy to techniques like
Microscopic Structures Image Analysis Metal, 3D Image (Red-Green) The microscopic methods range from dark field / bright field microscopy through polarisation- and inverse microscopy to techniques like
Timing Noise Measurement of High-Repetition-Rate Optical Pulses
 564 Timing Noise Measurement of High-Repetition-Rate Optical Pulses Hidemi Tsuchida National Institute of Advanced Industrial Science and Technology 1-1-1 Umezono, Tsukuba, 305-8568 JAPAN Tel: 81-29-861-5342;
564 Timing Noise Measurement of High-Repetition-Rate Optical Pulses Hidemi Tsuchida National Institute of Advanced Industrial Science and Technology 1-1-1 Umezono, Tsukuba, 305-8568 JAPAN Tel: 81-29-861-5342;
10. Phase Cycling and Pulsed Field Gradients Introduction to Phase Cycling - Quadrature images
 10. Phase Cycling and Pulsed Field Gradients 10.1 Introduction to Phase Cycling - Quadrature images The selection of coherence transfer pathways (CTP) by phase cycling or PFGs is the tool that allows the
10. Phase Cycling and Pulsed Field Gradients 10.1 Introduction to Phase Cycling - Quadrature images The selection of coherence transfer pathways (CTP) by phase cycling or PFGs is the tool that allows the
CHAPTER 7. Components of Optical Instruments
 CHAPTER 7 Components of Optical Instruments From: Principles of Instrumental Analysis, 6 th Edition, Holler, Skoog and Crouch. CMY 383 Dr Tim Laurens NB Optical in this case refers not only to the visible
CHAPTER 7 Components of Optical Instruments From: Principles of Instrumental Analysis, 6 th Edition, Holler, Skoog and Crouch. CMY 383 Dr Tim Laurens NB Optical in this case refers not only to the visible
Fast Raman Spectral Imaging Using Chirped Femtosecond Lasers
 Fast Raman Spectral Imaging Using Chirped Femtosecond Lasers Dan Fu 1, Gary Holtom 1, Christian Freudiger 1, Xu Zhang 2, Xiaoliang Sunney Xie 1 1. Department of Chemistry and Chemical Biology, Harvard
Fast Raman Spectral Imaging Using Chirped Femtosecond Lasers Dan Fu 1, Gary Holtom 1, Christian Freudiger 1, Xu Zhang 2, Xiaoliang Sunney Xie 1 1. Department of Chemistry and Chemical Biology, Harvard
Welcome to: LMBR Imaging Workshop. Imaging Fundamentals Mike Meade, Photometrics
 Welcome to: LMBR Imaging Workshop Imaging Fundamentals Mike Meade, Photometrics Introduction CCD Fundamentals Typical Cooled CCD Camera Configuration Shutter Optic Sealed Window DC Voltage Serial Clock
Welcome to: LMBR Imaging Workshop Imaging Fundamentals Mike Meade, Photometrics Introduction CCD Fundamentals Typical Cooled CCD Camera Configuration Shutter Optic Sealed Window DC Voltage Serial Clock
Confocal Microscopy. Kristin Jensen
 Confocal Microscopy Kristin Jensen 17.11.05 References Cell Biological Applications of Confocal Microscopy, Brian Matsumoto, chapter 1 Studying protein dynamics in living cells,, Jennifer Lippincott-Schwartz
Confocal Microscopy Kristin Jensen 17.11.05 References Cell Biological Applications of Confocal Microscopy, Brian Matsumoto, chapter 1 Studying protein dynamics in living cells,, Jennifer Lippincott-Schwartz
Transmission electron Microscopy
 Transmission electron Microscopy Image formation of a concave lens in geometrical optics Some basic features of the transmission electron microscope (TEM) can be understood from by analogy with the operation
Transmission electron Microscopy Image formation of a concave lens in geometrical optics Some basic features of the transmission electron microscope (TEM) can be understood from by analogy with the operation
Report on BLP Spectroscopy Experiments Conducted on October 6, 2017: M. Nansteel
 Report on BLP Spectroscopy Experiments Conducted on October 6, 2017: M. Nansteel Summary Several spectroscopic measurements were conducted on October 6, 2017 at BLP to characterize the radiant power of
Report on BLP Spectroscopy Experiments Conducted on October 6, 2017: M. Nansteel Summary Several spectroscopic measurements were conducted on October 6, 2017 at BLP to characterize the radiant power of
Photon Count. for Brainies.
 Page 1/12 Photon Count ounting for Brainies. 0. Preamble This document gives a general overview on InGaAs/InP, APD-based photon counting at telecom wavelengths. In common language, telecom wavelengths
Page 1/12 Photon Count ounting for Brainies. 0. Preamble This document gives a general overview on InGaAs/InP, APD-based photon counting at telecom wavelengths. In common language, telecom wavelengths
Period 3 Solutions: Electromagnetic Waves Radiant Energy II
 Period 3 Solutions: Electromagnetic Waves Radiant Energy II 3.1 Applications of the Quantum Model of Radiant Energy 1) Photon Absorption and Emission 12/29/04 The diagrams below illustrate an atomic nucleus
Period 3 Solutions: Electromagnetic Waves Radiant Energy II 3.1 Applications of the Quantum Model of Radiant Energy 1) Photon Absorption and Emission 12/29/04 The diagrams below illustrate an atomic nucleus
Solea. Supercontinuum Laser. Applications
 Solea Supercontinuum Laser Extended Spectral range: 525 nm - 900 nm (ECO mode), 480 nm - 900 nm (BOOST mode) Extended 2-year worldwide warranty* Supercontinuum output or wavelength selected output through
Solea Supercontinuum Laser Extended Spectral range: 525 nm - 900 nm (ECO mode), 480 nm - 900 nm (BOOST mode) Extended 2-year worldwide warranty* Supercontinuum output or wavelength selected output through
Spectral Analysis of the LUND/DMI Earthshine Telescope and Filters
 Spectral Analysis of the LUND/DMI Earthshine Telescope and Filters 12 August 2011-08-12 Ahmad Darudi & Rodrigo Badínez A1 1. Spectral Analysis of the telescope and Filters This section reports the characterization
Spectral Analysis of the LUND/DMI Earthshine Telescope and Filters 12 August 2011-08-12 Ahmad Darudi & Rodrigo Badínez A1 1. Spectral Analysis of the telescope and Filters This section reports the characterization
Energy in Photons. Light, Energy, and Electron Structure
 elearning 2009 Introduction Energy in Photons Light, Energy, and Electron Structure Publication No. 95007 Students often confuse the concepts of intensity of light and energy of light. This demonstration
elearning 2009 Introduction Energy in Photons Light, Energy, and Electron Structure Publication No. 95007 Students often confuse the concepts of intensity of light and energy of light. This demonstration
Imaging Retreat - UMASS Customized real-time confocal and 2-photon imaging
 Imaging Retreat - UMASS 2012 Customized real-time confocal and 2-photon imaging Mike Sanderson Department of Microbiology and Physiological Systems University of Massachusetts Medical School Thanks for
Imaging Retreat - UMASS 2012 Customized real-time confocal and 2-photon imaging Mike Sanderson Department of Microbiology and Physiological Systems University of Massachusetts Medical School Thanks for
LABORATÓRIUMI GYAKORLAT SILLABUSZ SYLLABUS OF A PRACTICAL DEMONSTRATION. financed by the program
 TÁMOP-4.1.1.C-13/1/KONV-2014-0001 projekt Az élettudományi-klinikai felsőoktatás gyakorlatorientált és hallgatóbarát korszerűsítése a vidéki képzőhelyek nemzetközi versenyképességének erősítésére program
TÁMOP-4.1.1.C-13/1/KONV-2014-0001 projekt Az élettudományi-klinikai felsőoktatás gyakorlatorientált és hallgatóbarát korszerűsítése a vidéki képzőhelyek nemzetközi versenyképességének erősítésére program
pco.edge 4.2 LT 0.8 electrons 2048 x 2048 pixel 40 fps : 1 > 70 % pco. low noise high resolution high speed high dynamic range
 edge 4.2 LT scientific CMOS camera high resolution 2048 x 2048 pixel low noise 0.8 electrons USB 3.0 small form factor high dynamic range 36 000 : 1 high speed 40 fps high quantum efficiency > 70 % edge
edge 4.2 LT scientific CMOS camera high resolution 2048 x 2048 pixel low noise 0.8 electrons USB 3.0 small form factor high dynamic range 36 000 : 1 high speed 40 fps high quantum efficiency > 70 % edge
Instruction manual and data sheet ipca h
 1/15 instruction manual ipca-21-05-1000-800-h Instruction manual and data sheet ipca-21-05-1000-800-h Broad area interdigital photoconductive THz antenna with microlens array and hyperhemispherical silicon
1/15 instruction manual ipca-21-05-1000-800-h Instruction manual and data sheet ipca-21-05-1000-800-h Broad area interdigital photoconductive THz antenna with microlens array and hyperhemispherical silicon
Upgrade to Andor s high-resolution Luca EM R EMCCD; the new price/performance benchmark.
 Features & benefits EMCCD Technology Ultimate in sensitivity from EMCCD gain. Even single photons are amplified above the noise. Full QE of the sensor is harnessed (visit www.emccd.com) Megapixel sensor
Features & benefits EMCCD Technology Ultimate in sensitivity from EMCCD gain. Even single photons are amplified above the noise. Full QE of the sensor is harnessed (visit www.emccd.com) Megapixel sensor
ZEISS Axiocam 503 color Your 3 Megapixel Microscope Camera for Fast Image Acquisition Fast, in True Color and Regular Field of View
 Product Information Version 1.0 ZEISS Axiocam 503 color Your 3 Megapixel Microscope Camera for Fast Image Acquisition Fast, in True Color and Regular Field of View ZEISS Axiocam 503 color Sensor Model
Product Information Version 1.0 ZEISS Axiocam 503 color Your 3 Megapixel Microscope Camera for Fast Image Acquisition Fast, in True Color and Regular Field of View ZEISS Axiocam 503 color Sensor Model
Zeiss 780 Training Notes
 Zeiss 780 Training Notes Turn on Main Switch, System PC and Components Switches 780 Start up sequence Do you need the argon laser (458, 488, 514 nm lines)? Yes Turn on the laser s main power switch and
Zeiss 780 Training Notes Turn on Main Switch, System PC and Components Switches 780 Start up sequence Do you need the argon laser (458, 488, 514 nm lines)? Yes Turn on the laser s main power switch and
pco.edge 4.2 LT 0.8 electrons 2048 x 2048 pixel 40 fps up to :1 up to 82 % pco. low noise high resolution high speed high dynamic range
 edge 4.2 LT scientific CMOS camera high resolution 2048 x 2048 pixel low noise 0.8 electrons USB 3.0 small form factor high dynamic range up to 37 500:1 high speed 40 fps high quantum efficiency up to
edge 4.2 LT scientific CMOS camera high resolution 2048 x 2048 pixel low noise 0.8 electrons USB 3.0 small form factor high dynamic range up to 37 500:1 high speed 40 fps high quantum efficiency up to
pco.edge electrons 2048 x 1536 pixel 50 fps :1 > 60 % pco. low noise high resolution high speed high dynamic range
 edge 3.1 scientific CMOS camera high resolution 2048 x 1536 pixel low noise 1.1 electrons global shutter USB 3.0 small form factor high dynamic range 27 000:1 high speed 50 fps high quantum efficiency
edge 3.1 scientific CMOS camera high resolution 2048 x 1536 pixel low noise 1.1 electrons global shutter USB 3.0 small form factor high dynamic range 27 000:1 high speed 50 fps high quantum efficiency
ECEN. Spectroscopy. Lab 8. copy. constituents HOMEWORK PR. Figure. 1. Layout of. of the
 ECEN 4606 Lab 8 Spectroscopy SUMMARY: ROBLEM 1: Pedrotti 3 12-10. In this lab, you will design, build and test an optical spectrum analyzer and use it for both absorption and emission spectroscopy. The
ECEN 4606 Lab 8 Spectroscopy SUMMARY: ROBLEM 1: Pedrotti 3 12-10. In this lab, you will design, build and test an optical spectrum analyzer and use it for both absorption and emission spectroscopy. The
Picosecond Time Analyzer Applications in...
 ORTEC AN52 Picosecond Time Analyzer Applications in... LIDAR and DIAL Time-of-Flight Mass Spectrometry Fluorescence/Phosphorescence Lifetime Spectrometry Pulse or Signal Jitter Analysis CONTENTS of this
ORTEC AN52 Picosecond Time Analyzer Applications in... LIDAR and DIAL Time-of-Flight Mass Spectrometry Fluorescence/Phosphorescence Lifetime Spectrometry Pulse or Signal Jitter Analysis CONTENTS of this
Terahertz spectroscopy measurements
 0 Terahertz spectroscopy measurements For general medicine and pharmacy students author: József Orbán, PhD. teaching facility: Univerity of Pécs, Medical School Department of Biophysics research facility:
0 Terahertz spectroscopy measurements For general medicine and pharmacy students author: József Orbán, PhD. teaching facility: Univerity of Pécs, Medical School Department of Biophysics research facility:
Dynamic Phase-Shifting Electronic Speckle Pattern Interferometer
 Dynamic Phase-Shifting Electronic Speckle Pattern Interferometer Michael North Morris, James Millerd, Neal Brock, John Hayes and *Babak Saif 4D Technology Corporation, 3280 E. Hemisphere Loop Suite 146,
Dynamic Phase-Shifting Electronic Speckle Pattern Interferometer Michael North Morris, James Millerd, Neal Brock, John Hayes and *Babak Saif 4D Technology Corporation, 3280 E. Hemisphere Loop Suite 146,
Sensors, Signals and Noise
 Sensors, Signals and Noise COURSE OUTLINE Introduction Signals and Noise Filtering Sensors: PD 4a -Photon Counting with PMTs Sergio Cova SENSORS SIGNALS AND NOISE Photodetectors 4a - PD4a rv 2015/01/05
Sensors, Signals and Noise COURSE OUTLINE Introduction Signals and Noise Filtering Sensors: PD 4a -Photon Counting with PMTs Sergio Cova SENSORS SIGNALS AND NOISE Photodetectors 4a - PD4a rv 2015/01/05
Wavelength Control and Locking with Sub-MHz Precision
 Wavelength Control and Locking with Sub-MHz Precision A PZT actuator on one of the resonator mirrors enables the Verdi output wavelength to be rapidly tuned over a range of several GHz or tightly locked
Wavelength Control and Locking with Sub-MHz Precision A PZT actuator on one of the resonator mirrors enables the Verdi output wavelength to be rapidly tuned over a range of several GHz or tightly locked
8.5 Modulation of Signals
 8.5 Modulation of Signals basic idea and goals measuring atomic absorption without modulation measuring atomic absorption with modulation the tuned amplifier, diode rectifier and low pass the lock-in amplifier
8.5 Modulation of Signals basic idea and goals measuring atomic absorption without modulation measuring atomic absorption with modulation the tuned amplifier, diode rectifier and low pass the lock-in amplifier
visibility values: 1) V1=0.5 2) V2=0.9 3) V3=0.99 b) In the three cases considered, what are the values of FSR (Free Spectral Range) and
 EXERCISES OF OPTICAL MEASUREMENTS BY ENRICO RANDONE AND CESARE SVELTO EXERCISE 1 A CW laser radiation (λ=2.1 µm) is delivered to a Fabry-Pérot interferometer made of 2 identical plane and parallel mirrors
EXERCISES OF OPTICAL MEASUREMENTS BY ENRICO RANDONE AND CESARE SVELTO EXERCISE 1 A CW laser radiation (λ=2.1 µm) is delivered to a Fabry-Pérot interferometer made of 2 identical plane and parallel mirrors
Dynamic Phase-Shifting Microscopy Tracks Living Cells
 from photonics.com: 04/01/2012 http://www.photonics.com/article.aspx?aid=50654 Dynamic Phase-Shifting Microscopy Tracks Living Cells Dr. Katherine Creath, Goldie Goldstein and Mike Zecchino, 4D Technology
from photonics.com: 04/01/2012 http://www.photonics.com/article.aspx?aid=50654 Dynamic Phase-Shifting Microscopy Tracks Living Cells Dr. Katherine Creath, Goldie Goldstein and Mike Zecchino, 4D Technology
Rates of excitation, emission, ISC
 Bi177 Lecture 4 Fluorescence Microscopy Phenomenon of Fluorescence Energy Diagram Rates of excitation, emission, ISC Practical Issues Lighting, Filters More on diffraction Point Spread Functions Thus Far,
Bi177 Lecture 4 Fluorescence Microscopy Phenomenon of Fluorescence Energy Diagram Rates of excitation, emission, ISC Practical Issues Lighting, Filters More on diffraction Point Spread Functions Thus Far,
By Pierre Olivier, Vice President, Engineering and Manufacturing, LeddarTech Inc.
 Leddar optical time-of-flight sensing technology, originally discovered by the National Optics Institute (INO) in Quebec City and developed and commercialized by LeddarTech, is a unique LiDAR technology
Leddar optical time-of-flight sensing technology, originally discovered by the National Optics Institute (INO) in Quebec City and developed and commercialized by LeddarTech, is a unique LiDAR technology
Point Spread Function Estimation Tool, Alpha Version. A Plugin for ImageJ
 Tutorial Point Spread Function Estimation Tool, Alpha Version A Plugin for ImageJ Benedikt Baumgartner Jo Helmuth jo.helmuth@inf.ethz.ch MOSAIC Lab, ETH Zurich www.mosaic.ethz.ch This tutorial explains
Tutorial Point Spread Function Estimation Tool, Alpha Version A Plugin for ImageJ Benedikt Baumgartner Jo Helmuth jo.helmuth@inf.ethz.ch MOSAIC Lab, ETH Zurich www.mosaic.ethz.ch This tutorial explains
Leica TCS SP8 Quick Start Guide
 Leica TCS SP8 Quick Start Guide Leica TCS SP8 System Overview Start-Up Procedure 1. Turn on the CTR Control Box, Fluorescent Light for the microscope stand. 2. Turn on the Scanner Power (1) on the front
Leica TCS SP8 Quick Start Guide Leica TCS SP8 System Overview Start-Up Procedure 1. Turn on the CTR Control Box, Fluorescent Light for the microscope stand. 2. Turn on the Scanner Power (1) on the front
Εισαγωγική στην Οπτική Απεικόνιση
 Εισαγωγική στην Οπτική Απεικόνιση Δημήτριος Τζεράνης, Ph.D. Εμβιομηχανική και Βιοϊατρική Τεχνολογία Τμήμα Μηχανολόγων Μηχανικών Ε.Μ.Π. Χειμερινό Εξάμηνο 2015 Light: A type of EM Radiation EM radiation:
Εισαγωγική στην Οπτική Απεικόνιση Δημήτριος Τζεράνης, Ph.D. Εμβιομηχανική και Βιοϊατρική Τεχνολογία Τμήμα Μηχανολόγων Μηχανικών Ε.Μ.Π. Χειμερινό Εξάμηνο 2015 Light: A type of EM Radiation EM radiation:
SC5407A/SC5408A 100 khz to 6 GHz RF Upconverter. Datasheet. Rev SignalCore, Inc.
 SC5407A/SC5408A 100 khz to 6 GHz RF Upconverter Datasheet Rev 1.2 2017 SignalCore, Inc. support@signalcore.com P R O D U C T S P E C I F I C A T I O N S Definition of Terms The following terms are used
SC5407A/SC5408A 100 khz to 6 GHz RF Upconverter Datasheet Rev 1.2 2017 SignalCore, Inc. support@signalcore.com P R O D U C T S P E C I F I C A T I O N S Definition of Terms The following terms are used
225 Lock-in Amplifier
 225 Lock-in Amplifier 225.02 Bentham Instruments Ltd 1 2 Bentham Instruments Ltd 225.02 1. WHAT IS A LOCK-IN? There are a number of ways of visualising the operation and significance of a lock-in amplifier.
225 Lock-in Amplifier 225.02 Bentham Instruments Ltd 1 2 Bentham Instruments Ltd 225.02 1. WHAT IS A LOCK-IN? There are a number of ways of visualising the operation and significance of a lock-in amplifier.
Development of a High-speed Super-resolution Confocal Scanner
 Development of a High-speed Super-resolution Confocal Scanner Takuya Azuma *1 Takayuki Kei *1 Super-resolution microscopy techniques that overcome the spatial resolution limit of conventional light microscopy
Development of a High-speed Super-resolution Confocal Scanner Takuya Azuma *1 Takayuki Kei *1 Super-resolution microscopy techniques that overcome the spatial resolution limit of conventional light microscopy
Image Capture TOTALLAB
 1 Introduction In order for image analysis to be performed on a gel or Western blot, it must first be converted into digital data. Good image capture is critical to guarantee optimal performance of automated
1 Introduction In order for image analysis to be performed on a gel or Western blot, it must first be converted into digital data. Good image capture is critical to guarantee optimal performance of automated
High-Resolution Bubble Printing of Quantum Dots
 SUPPORTING INFORMATION High-Resolution Bubble Printing of Quantum Dots Bharath Bangalore Rajeeva 1, Linhan Lin 1, Evan P. Perillo 2, Xiaolei Peng 1, William W. Yu 3, Andrew K. Dunn 2, Yuebing Zheng 1,*
SUPPORTING INFORMATION High-Resolution Bubble Printing of Quantum Dots Bharath Bangalore Rajeeva 1, Linhan Lin 1, Evan P. Perillo 2, Xiaolei Peng 1, William W. Yu 3, Andrew K. Dunn 2, Yuebing Zheng 1,*
Determining MTF with a Slant Edge Target ABSTRACT AND INTRODUCTION
 Determining MTF with a Slant Edge Target Douglas A. Kerr Issue 2 October 13, 2010 ABSTRACT AND INTRODUCTION The modulation transfer function (MTF) of a photographic lens tells us how effectively the lens
Determining MTF with a Slant Edge Target Douglas A. Kerr Issue 2 October 13, 2010 ABSTRACT AND INTRODUCTION The modulation transfer function (MTF) of a photographic lens tells us how effectively the lens
Supplementary Figures
 1 Supplementary Figures a) f rep,1 Δf f rep,2 = f rep,1 +Δf RF Domain Optical Domain b) Aliasing region Supplementary Figure 1. Multi-heterdoyne beat note of two slightly shifted frequency combs. a Case
1 Supplementary Figures a) f rep,1 Δf f rep,2 = f rep,1 +Δf RF Domain Optical Domain b) Aliasing region Supplementary Figure 1. Multi-heterdoyne beat note of two slightly shifted frequency combs. a Case
Components of Optical Instruments
 Components of Optical Instruments General Design of Optical Instruments Sources of Radiation Wavelength Selectors (Filters, Monochromators, Interferometers) Sample Containers Radiation Transducers (Detectors)
Components of Optical Instruments General Design of Optical Instruments Sources of Radiation Wavelength Selectors (Filters, Monochromators, Interferometers) Sample Containers Radiation Transducers (Detectors)
Confocal, hyperspectral, spinning disk
 Confocal, hyperspectral, spinning disk Administrative HW 6 due on Fri Midterm on Wed Covers everything since previous midterm 8.5 x 11 sheet allowed, 1 side Guest lecture by Joe Dragavon on Mon 10/30 Last
Confocal, hyperspectral, spinning disk Administrative HW 6 due on Fri Midterm on Wed Covers everything since previous midterm 8.5 x 11 sheet allowed, 1 side Guest lecture by Joe Dragavon on Mon 10/30 Last
Chemical Imaging. Whiskbroom Imaging. Staring Imaging. Pushbroom Imaging. Whiskbroom. Staring. Pushbroom
 Chemical Imaging Whiskbroom Chemical Imaging (CI) combines different technologies like optical microscopy, digital imaging and molecular spectroscopy in combination with multivariate data analysis methods.
Chemical Imaging Whiskbroom Chemical Imaging (CI) combines different technologies like optical microscopy, digital imaging and molecular spectroscopy in combination with multivariate data analysis methods.
C1587 UNIVERSAL STREAK CAMERA Selectable features to suit a variety of applications from the vacuum ultraviolet through the near infrared.
 C1587 UNIVERSAL STREAK CAMERA Selectable features to suit a variety of applications from the vacuum ultraviolet through the near infrared. HAMAMATSU 1.515 t,5!l 1.525 1.5» UftVCLCMCTH (RICIOKTCO A Measurement
C1587 UNIVERSAL STREAK CAMERA Selectable features to suit a variety of applications from the vacuum ultraviolet through the near infrared. HAMAMATSU 1.515 t,5!l 1.525 1.5» UftVCLCMCTH (RICIOKTCO A Measurement
Lab4 Hanbury Brown and Twiss Setup. Photon Antibunching
 Lab4 Hanbury Brown and Twiss Setup. Photon Antibunching Shule Li Abstract Antibunching is a purely quantum effect and cannot be realized from the classical theory of light. By observing the antibunching
Lab4 Hanbury Brown and Twiss Setup. Photon Antibunching Shule Li Abstract Antibunching is a purely quantum effect and cannot be realized from the classical theory of light. By observing the antibunching
Non-linear Control. Part III. Chapter 8
 Chapter 8 237 Part III Chapter 8 Non-linear Control The control methods investigated so far have all been based on linear feedback control. Recently, non-linear control techniques related to One Cycle
Chapter 8 237 Part III Chapter 8 Non-linear Control The control methods investigated so far have all been based on linear feedback control. Recently, non-linear control techniques related to One Cycle
LlIGHT REVIEW PART 2 DOWNLOAD, PRINT and submit for 100 points
 WRITE ON SCANTRON WITH NUMBER 2 PENCIL DO NOT WRITE ON THIS TEST LlIGHT REVIEW PART 2 DOWNLOAD, PRINT and submit for 100 points Multiple Choice Identify the choice that best completes the statement or
WRITE ON SCANTRON WITH NUMBER 2 PENCIL DO NOT WRITE ON THIS TEST LlIGHT REVIEW PART 2 DOWNLOAD, PRINT and submit for 100 points Multiple Choice Identify the choice that best completes the statement or
Advanced Optical Communications Prof. R. K. Shevgaonkar Department of Electrical Engineering Indian Institute of Technology, Bombay
 Advanced Optical Communications Prof. R. K. Shevgaonkar Department of Electrical Engineering Indian Institute of Technology, Bombay Lecture No. # 27 EDFA In the last lecture, we talked about wavelength
Advanced Optical Communications Prof. R. K. Shevgaonkar Department of Electrical Engineering Indian Institute of Technology, Bombay Lecture No. # 27 EDFA In the last lecture, we talked about wavelength
Add CLUE to your SEM. High-efficiency CL signal-collection. Designed for your SEM and application. Maintains original SEM functionality
 Add CLUE to your SEM Designed for your SEM and application The CLUE family offers dedicated CL systems for imaging and spectroscopic analysis suitable for most SEMs. In addition, when combined with other
Add CLUE to your SEM Designed for your SEM and application The CLUE family offers dedicated CL systems for imaging and spectroscopic analysis suitable for most SEMs. In addition, when combined with other
Kit for building your own THz Time-Domain Spectrometer
 Kit for building your own THz Time-Domain Spectrometer 16/06/2016 1 Table of contents 0. Parts for the THz Kit... 3 1. Delay line... 4 2. Pulse generator and lock-in detector... 5 3. THz antennas... 6
Kit for building your own THz Time-Domain Spectrometer 16/06/2016 1 Table of contents 0. Parts for the THz Kit... 3 1. Delay line... 4 2. Pulse generator and lock-in detector... 5 3. THz antennas... 6
