What impact have these advances had on the time resolution of affordable wide-field fluorescence microscopes?
|
|
|
- Daniela Long
- 6 years ago
- Views:
Transcription
1 High-speed acquisition of multi-wavelength fluorescence images: an interview with Jeremy Graham, Cairn Research Interview conducted by April Cashin-Garbutt, MA (Cantab) Please can you give an overview of the recent advances in Electron Multiplying Charge Coupled Device (EMCCD) and Complementary Metal Oxide Silicon (CMOS) camera technologies? On-chip Electron Multiplication is now a mature technology, which has allowed manufacturers to build cameras that are both fast and sensitive; ideal for live cell fluorescence imaging. The central concept is that the photon signal from each pixel is amplified greatly prior to digitization, thus reducing read noise (analogous to a photomultiplier or image intensifier, but with higher inherent sensitivity (Quantum Efficiency)). Recently there have been useful speed improvements, both in respect to the clock speed of the full sensor and the ability to read sub-regions more rapidly by optically masking the required field of view and using the dark pixels for temporary storage. At Cairn we have addressed this by designing suitable mechanical masks our 'OptoMask' product range. Scientific CMOS cameras on the other hand, fundamentally operate at MUCH faster clock speeds with many more and smaller pixels and the challenge has been to get the noise and sensitivity comparable, or better than CCD. Modern chip design, with on-board processing, correlated double sampling (CDS) and advanced microlensing has made this a reality. However, when using these cameras an important consideration is that they can generate data at up to 1Gb per second, requiring serious computing power both to capture the data to RAM or hard disk and for subsequent processing. The large sensor size also pushes the field of view limits of today s research microscopes; care needs to be taken to achieve flat field illumination. What impact have these advances had on the time resolution of affordable wide-field fluorescence microscopes? Inexpensive scientific CMOS cameras have largely replaced interline CCD cameras for dynamic live-cell imaging. A fast high-end interline CCD camera can acquire images of around one million pixels at 10 to 15 full frames per second. Scientific CMOS cameras have equivalent pixel size, but much larger sensors and can acquire images of more than four megapixels at 100 frames per second. The speed improvement for a given field of view is greater than an order of magnitude! EMCCDs are also now offering high temporal resolution, with frame rates of over 60 frames/sec at 512x512 pixels and over 500 frames/sec at 128x128 pixels.
2 There are many important differences between the two technologies involving rolling shutters, multiplication noise, microlenses, digitisation, FPGA processing, Niquist resolution, and well-depth amongst others. The ultimate decision between EMCCD and scientific CMOS for a given application is fascinating and now depends rather directly on the number of photons that the sample can withstand without damage. Scientific CMOS is better for most applications, but there is still a significant sub-set of low-light and high-speed applications where the EMCCD is preferred. In practise, this subset seems to be rather larger than simple noise calculations would suggest; my suspicion is that the benefits of a high native fill factor and low-noise amplification tend to be overlooked. Please contact us if you want advice for your specific application, we work with all the main companies and strive to be impartial. What problems can occur when trying to realise these benefits when using multiple dyes or genetic markers? Biologists often want to use indicators of different wavelength in the same experiment. They may be using several markers to look at different features of their cells, ratio imaging intracellular calcium, or studying colocalisation using FRET. Living samples move, in many cases rapidly, so for accurate overlay the different channels should be acquired at the same time. The traditional method of multicolour fluorescence imaging has been to take sequential images with the illumination and detection light changed mechanically between frames using stepping filter wheels or revolving carousels. These devices have significant inertia and are inherently slow; fine for fixed slides, but not good for live cells. For example, to perform two-colour imaging on a fast camera, you are practically limited to ~5 to 10 frames per second, even though the camera is capable of achieving TEN times better than this. Mechanical delays and the requirement for (serial) software timing have negated much of the benefit of the improvements in camera technology! How can these problems be overcome? On the illumination side, the timing delays can be overcome using solid-state LED and laser light sources that can be electronically modulated in real time with no mechanical movement. Many research cameras will generate the necessary synchronisation signals, or companies such as Cairn can help out with dedicated timing boxes. Multi-wavelength detection, for example to look at green and red fluorescent proteins with good time resolution, is more difficult. One useful approach is to use multiple cameras or to use multiple regions of the same camera differentiated with spectral beamsplitters to detect different wavelengths simultaneously.
3 Spectral beamsplitters The Cairn MultiCam allows up to four cameras to image the same field of view simultaneously at different wavelengths. The Cairn OptoSplit II allows simultaneous dual-channel imaging on the same camera sensor with half the field of view. These are both very powerful solutions being truly simultaneous and having no moving parts. However, you either sacrifice the field of view by splitting a smaller region of interest across a single camera sensor, or add cost and complexity by running more than one camera, especially if you want things to be well synchronized.
4 A further problem is that the cameras / regions are exposed to light at all times, so there is the potential for crosstalk between illumination channels. This is an important consideration and will usually result in images with higher background than if only one illumination and emission wavelength were used at a time. How could a continuously spinning filter wheel be used to help? By synchronising a continuously spinning emission filter wheel with multi-colour modulatable light sources, it is possible to operate EMCCD or scientific CMOS cameras at very high frame rates whilst taking sequential multi-channel images. Spinning, rather than stepping, avoids the need for repeated rapid acceleration and deceleration cycles and the consequent delays and mechanical vibration. By synchronising both the camera and lightsource to the spinning wheel it is possible to perform fast, robust multi-wavelength imaging without making any demands of the software. The camera will simply produce an interleaved stack which can be demultiplexed off-line in a few seconds. The Cairn OptoSpin fast stepping and spinning filter wheel generates all the timing signals for fast imaging at up to six different wavelengths (example shows a three colour protocol). The time resolution of the camera is fully exploited for three colour imaging without the cost of an emission splitter, multiple cameras or commercial software.
5 How can crosstalk be removed in simultaneous multichannel imaging? Although fast, the images shown above are still sequential, so how can we go faster still? If measurements need to be truly simultaneous, then the light does need to be separated with spectral beam splitters (dichroic mirrors). The problem, as discussed earlier, is that inevitably absorption light for one fluorophore will interfere with the emission wavelength of a different fluorophore. For example, we have a sample that is expressed with green and red fluorescent proteins, requiring blue and yellow excitation light respectively. When the green protein is excited with blue light, it will not only emit green fluorescence, but also some component of the same structure will bleed-through into the red channel. Now, imagine if the fluorescence of the green protein is much stronger than the red, then the crosstalk into the red channel from GFP could mask the much lower (desired) signal from the red fluorophore (RFP). The only way to avoid this is to ensure that when the blue light is on, photons only reach the green detector area and that when the yellow light is on, photons only reach the red detector area, so there's no crosscontamination between the two. That's very straight forward using conventional slow methods, because you would step in the appropriate illumination and detection filters between each measurement. The problem is if two or more emission filters are continuously in the light path, then you can t avoid this contamination. Our fix is to synchronise a continuously spinning filter wheel with the camera so that different emission channels are in the light path sequentially within a single exposure. If the excitation light sources are modulated in sync with the emission wheel, then we can produce framerate images at different wavelengths with no crosstalk. This is analogous to strobe photography where the exposure is determined by the illumination and not by the camera integration. The same trick holds for different splitter configurations or multi-camera set ups. The left hand image shows significant crosstalk which is not evident on the right.
6 The take home message is that if you want to enjoy the benefits of modern high-speed cameras for multichannel imaging then there are solutions, they are not expensive and do not require 'fancy' software. What do you think the future holds for fluorescence imaging? I think that fluorescence imaging will continue to evolve as a powerful tool for biological research and biomedical analysis. Cheaper and more portable devices are likely to become available for routine medical assays at the point of use, particularly in developing economies. For high-end imaging one important recent area of improvement relates to the size of the smallest structures that can be accurately measured. This resolution limit for light microscopy is solely a function of wavelength and the Numerical Aperture of the objective, with visible light this works out to around 200 nanometers. Unfortunately, some biologists wish to image very small structures and require better resolution. Until fairly recently this required an Electron Microscopy, which is not friendly for live samples. Fortunately, a number of biophysicists have been working on this problem for some time and in 2013, three groups were jointly awarded the Nobel Prize for Chemistry for pioneering three different methodologies (tricks) to overcome the diffraction limit, and resolve structures down to tens of nanometers. The next challenge is to bring these, so called, Super Resolution techniques further into the live cell domain, by improving both the temporal resolution and by making the instruments and sample preparations simpler and therefore accessible to more groups. The experiments now being conducted by the most advanced and best funded researchers are already very impressive. Another area which is likely to progress in the next few years is that routine fluorescence measurements will be better spectrally resolved, both with more fluorophores being used simultaneously and also with better differentiation of the signal of interest from general background fluorescence. Cameras are already available with larger sensors than can be used with commercial microscopes, so there are possibilities to use the additional pixels to extract more information, for example real-time simultaneous spatial and spectral imaging. Although fluorescence measurements are relatively non-invasive compared with alternative techniques, there has recently been a welcome emphasis on reducing the illumination dosages as far as possible to protect both the fluorophores and the biological preparations from photo damage. Also to, hopefully, ensure that the behaviour of the tissue or cells is physiologically relevant and not just a reaction to being bombarded with photons! Techniques such as Single Plane Illumination Microscopy (SPIM), which is inherently less photo damaging, are likely to become more and more prevalent. Here, instead of the illumination light coming through the objective, it actually comes from the side of the sample as a single plane. The advantage of this is that you are only illuminating the focal plane of the camera, not everything else. By translating the sample through the focal plane, a 3D image can be constructed with significantly reduced photon exposure. What are Cairn Research s plans for the future? Our plan is to continue to exploit new, fun, technology to develop (hopefully) innovative products and solutions for fluorescence microscopy and macroscopy. Microscope illumination is a very competitive area, but we have several niche products in development using dedicated LEDs for fast calcium measurements and optogenetics activation. Laser diodes are getting
7 cheaper, more reliable and more powerful all the time and we are collaborating with 89 North ( on a very exciting illuminator which should set the new gold standard for many fluorescence and related applications which require intense illumination from a small (point) source. On the detection side we already have a wide range of accessories encompassing the image splitters, multicamera adapters and filter wheel mentioned above. We plan to extend this range for simultaneous multifocal depth imaging and to make use of new (larger) cameras as they become available. We are also collaborating on a novel implementation of camera technology which allows more detailed interrogation of the detected signal. This will hopefully prove to be of benefit in Super Resolution microscopy and particle tracking. As well as designing and building off-the-shelf products we like to offer turnkey integrated imaging systems and custom designed solutions to answer novel questions which cannot be fully addressed with existing instruments. Historically, many of our bespoke custom designs have evolved into products in their own right and become part of our turnkey product or integrated system ranges. Long may this continue! We are now looking at making higher-end imaging systems affordable to more groups; putting together systems and sub-systems making use of new technology, but at a price point where individual groups can use it rather than requiring core facilities. To this end we are developing a stand-alone TIRF / Super Resolution illuminator that is independent of the software and hardware platform and can work with inexpensive, incoherent, light sources. Finally, we are very keen to do our bit to help make fluorescence experiments less phototoxic and more biologically relevant, and are addressing this issue, by developing new instruments in an attempt to change the way in which experiments are conducted. As a starting point we feel that there is a need for a simple affordable Single Plane (light sheet) Illuminator.; commercial systems are inflexible and expensive, so many groups are now having to build their own. Selfbuild may be fun, but it is time consuming and is limiting the uptake of a very useful and important technique. We hope that our (soon to be released), light sheet illuminator will help to address this; results with the early prototypes have been extremely promising.
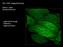
8 The growing edge of a reef-building coral, imaged using a Cairn prototype low-cost lightsheet fluorescence microscope. Live imaging helps us understand how climate change affects the growth of these beautiful organisms. However, since they are easily damaged by high light intensities, a special low-light approach using pulsed lightsheets is required. As a self-financed company with no external shareholders, independence is a key theme in our strategy and philosophy. This allows us to work in very close partnership with researchers and with other 'techie' companies; we greatly value these interactions so please don t hesitate to contact us with ideas or requirements our ears and eyes are always open! Where can readers find more information? They can visit our website or contact us. Cairn Research Ltd Graveney Road Faversham Kent, ME13 8UP UK Tel: + 44 (0) Fax: + 44 (0) sales@cairn-research.co.uk tech@cairn-research.co.uk
9 About Jeremy Graham With over twenty years of experience in live-cell microscopy, Jeremy Graham has worked in various technical and sales roles within Cairn Research, culminating in being appointed Managing Director in During his time as Managing Director he has helped the business to quadruple in size expanding into many new areas of fluorescence microscopy and related imaging. He has played a key role in the development of the majority of the products and systems in the Cairn portfolio and has enjoyed successful collaborations with leading researchers in many of the world s most prestigious research institutes. He regularly contributes to international teaching courses, has been a consultant on several research panels and has contributed to the scientific literature. Jeremy s role is largely concerned with identifying emerging fields of scientific interest and helping to connect these with new technology to develop novel products and solutions.
Welcome to: LMBR Imaging Workshop. Imaging Fundamentals Mike Meade, Photometrics
 Welcome to: LMBR Imaging Workshop Imaging Fundamentals Mike Meade, Photometrics Introduction CCD Fundamentals Typical Cooled CCD Camera Configuration Shutter Optic Sealed Window DC Voltage Serial Clock
Welcome to: LMBR Imaging Workshop Imaging Fundamentals Mike Meade, Photometrics Introduction CCD Fundamentals Typical Cooled CCD Camera Configuration Shutter Optic Sealed Window DC Voltage Serial Clock
Opterra II Multipoint Scanning Confocal Microscope. Innovation with Integrity
 Opterra II Multipoint Scanning Confocal Microscope Enabling 4D Live-Cell Fluorescence Imaging through Speed, Sensitivity, Viability and Simplicity Innovation with Integrity Fluorescence Microscopy The
Opterra II Multipoint Scanning Confocal Microscope Enabling 4D Live-Cell Fluorescence Imaging through Speed, Sensitivity, Viability and Simplicity Innovation with Integrity Fluorescence Microscopy The
Upgrade to Andor s high-resolution Luca EM R EMCCD; the new price/performance benchmark.
 Features & benefits EMCCD Technology Ultimate in sensitivity from EMCCD gain. Even single photons are amplified above the noise. Full QE of the sensor is harnessed (visit www.emccd.com) Megapixel sensor
Features & benefits EMCCD Technology Ultimate in sensitivity from EMCCD gain. Even single photons are amplified above the noise. Full QE of the sensor is harnessed (visit www.emccd.com) Megapixel sensor
Detectors for microscopy - CCDs, APDs and PMTs. Antonia Göhler. Nov 2014
 Detectors for microscopy - CCDs, APDs and PMTs Antonia Göhler Nov 2014 Detectors/Sensors in general are devices that detect events or changes in quantities (intensities) and provide a corresponding output,
Detectors for microscopy - CCDs, APDs and PMTs Antonia Göhler Nov 2014 Detectors/Sensors in general are devices that detect events or changes in quantities (intensities) and provide a corresponding output,
Why and How? Daniel Gitler Dept. of Physiology Ben-Gurion University of the Negev. Microscopy course, Michmoret Dec 2005
 Why and How? Daniel Gitler Dept. of Physiology Ben-Gurion University of the Negev Why use confocal microscopy? Principles of the laser scanning confocal microscope. Image resolution. Manipulating the
Why and How? Daniel Gitler Dept. of Physiology Ben-Gurion University of the Negev Why use confocal microscopy? Principles of the laser scanning confocal microscope. Image resolution. Manipulating the
Camera Test Protocol. Introduction TABLE OF CONTENTS. Camera Test Protocol Technical Note Technical Note
 Technical Note CMOS, EMCCD AND CCD CAMERAS FOR LIFE SCIENCES Camera Test Protocol Introduction The detector is one of the most important components of any microscope system. Accurate detector readings
Technical Note CMOS, EMCCD AND CCD CAMERAS FOR LIFE SCIENCES Camera Test Protocol Introduction The detector is one of the most important components of any microscope system. Accurate detector readings
Multi-channel imaging cytometry with a single detector
 Multi-channel imaging cytometry with a single detector Sarah Locknar 1, John Barton 1, Mark Entwistle 2, Gary Carver 1 and Robert Johnson 1 1 Omega Optical, Brattleboro, VT 05301 2 Philadelphia Lightwave,
Multi-channel imaging cytometry with a single detector Sarah Locknar 1, John Barton 1, Mark Entwistle 2, Gary Carver 1 and Robert Johnson 1 1 Omega Optical, Brattleboro, VT 05301 2 Philadelphia Lightwave,
Please do not hesitate to contact us if you have any questions or issues during installation or operation
 OPTOSPLIT II Manual BYPASS This guide details initial set up and installation of your OptoSplit II Bypass (BP) image splitter. Each unit is serial numbered, calibrated and QC d prior to delivery, therefore
OPTOSPLIT II Manual BYPASS This guide details initial set up and installation of your OptoSplit II Bypass (BP) image splitter. Each unit is serial numbered, calibrated and QC d prior to delivery, therefore
3. are adherent cells (ie. cells in suspension are too far away from the coverslip)
 Before you begin, make sure your sample... 1. is seeded on #1.5 coverglass (thickness = 0.17) 2. is an aqueous solution (ie. fixed samples mounted on a slide will not work - not enough difference in refractive
Before you begin, make sure your sample... 1. is seeded on #1.5 coverglass (thickness = 0.17) 2. is an aqueous solution (ie. fixed samples mounted on a slide will not work - not enough difference in refractive
Practical work no. 3: Confocal Live Cell Microscopy
 Practical work no. 3: Confocal Live Cell Microscopy Course Instructor: Mikko Liljeström (MIU) 1 Background Confocal microscopy: The main idea behind confocality is that it suppresses the signal outside
Practical work no. 3: Confocal Live Cell Microscopy Course Instructor: Mikko Liljeström (MIU) 1 Background Confocal microscopy: The main idea behind confocality is that it suppresses the signal outside
Development of a High-speed Super-resolution Confocal Scanner
 Development of a High-speed Super-resolution Confocal Scanner Takuya Azuma *1 Takayuki Kei *1 Super-resolution microscopy techniques that overcome the spatial resolution limit of conventional light microscopy
Development of a High-speed Super-resolution Confocal Scanner Takuya Azuma *1 Takayuki Kei *1 Super-resolution microscopy techniques that overcome the spatial resolution limit of conventional light microscopy
Multifluorescence The Crosstalk Problem and Its Solution
 Multifluorescence The Crosstalk Problem and Its Solution If a specimen is labeled with more than one fluorochrome, each image channel should only show the emission signal of one of them. If, in a specimen
Multifluorescence The Crosstalk Problem and Its Solution If a specimen is labeled with more than one fluorochrome, each image channel should only show the emission signal of one of them. If, in a specimen
Examination, TEN1, in courses SK2500/SK2501, Physics of Biomedical Microscopy,
 KTH Applied Physics Examination, TEN1, in courses SK2500/SK2501, Physics of Biomedical Microscopy, 2009-06-05, 8-13, FB51 Allowed aids: Compendium Imaging Physics (handed out) Compendium Light Microscopy
KTH Applied Physics Examination, TEN1, in courses SK2500/SK2501, Physics of Biomedical Microscopy, 2009-06-05, 8-13, FB51 Allowed aids: Compendium Imaging Physics (handed out) Compendium Light Microscopy
Training Guide for Carl Zeiss LSM 510 META Confocal Microscope
 Training Guide for Carl Zeiss LSM 510 META Confocal Microscope AIM 4.2 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Turn ON Components and System/PC switches
Training Guide for Carl Zeiss LSM 510 META Confocal Microscope AIM 4.2 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Turn ON Components and System/PC switches
The new CMOS Tracking Camera used at the Zimmerwald Observatory
 13-0421 The new CMOS Tracking Camera used at the Zimmerwald Observatory M. Ploner, P. Lauber, M. Prohaska, P. Schlatter, J. Utzinger, T. Schildknecht, A. Jaeggi Astronomical Institute, University of Bern,
13-0421 The new CMOS Tracking Camera used at the Zimmerwald Observatory M. Ploner, P. Lauber, M. Prohaska, P. Schlatter, J. Utzinger, T. Schildknecht, A. Jaeggi Astronomical Institute, University of Bern,
High Resolution BSI Scientific CMOS
 CMOS, EMCCD AND CCD CAMERAS FOR LIFE SCIENCES High Resolution BSI Scientific CMOS Prime BSI delivers the perfect balance between high resolution imaging and sensitivity with an optimized pixel design and
CMOS, EMCCD AND CCD CAMERAS FOR LIFE SCIENCES High Resolution BSI Scientific CMOS Prime BSI delivers the perfect balance between high resolution imaging and sensitivity with an optimized pixel design and
e2v Launches New Onyx 1.3M for Premium Performance in Low Light Conditions
 e2v Launches New Onyx 1.3M for Premium Performance in Low Light Conditions e2v s Onyx family of image sensors is designed for the most demanding outdoor camera and industrial machine vision applications,
e2v Launches New Onyx 1.3M for Premium Performance in Low Light Conditions e2v s Onyx family of image sensors is designed for the most demanding outdoor camera and industrial machine vision applications,
 www.cairn-research.co.uk Free Phone: 08453301267 (UK only) Tel: +44 (0) 1795590140 Fax: +44 (0) 1795594510 Important Information Please Read Before Installing and Operating Your Optosplit II For maximum
www.cairn-research.co.uk Free Phone: 08453301267 (UK only) Tel: +44 (0) 1795590140 Fax: +44 (0) 1795594510 Important Information Please Read Before Installing and Operating Your Optosplit II For maximum
Imaging Retreat - UMASS Customized real-time confocal and 2-photon imaging
 Imaging Retreat - UMASS 2012 Customized real-time confocal and 2-photon imaging Mike Sanderson Department of Microbiology and Physiological Systems University of Massachusetts Medical School Thanks for
Imaging Retreat - UMASS 2012 Customized real-time confocal and 2-photon imaging Mike Sanderson Department of Microbiology and Physiological Systems University of Massachusetts Medical School Thanks for
Shreyash Tandon M.S. III Year
 Shreyash Tandon M.S. III Year 20091015 Confocal microscopy is a powerful tool for generating high-resolution images and 3-D reconstructions of a specimen by using point illumination and a spatial pinhole
Shreyash Tandon M.S. III Year 20091015 Confocal microscopy is a powerful tool for generating high-resolution images and 3-D reconstructions of a specimen by using point illumination and a spatial pinhole
Leica TCS SP8 Quick Start Guide
 Leica TCS SP8 Quick Start Guide Leica TCS SP8 System Overview Start-Up Procedure 1. Turn on the CTR Control Box, Fluorescent Light for the microscope stand. 2. Turn on the Scanner Power (1) on the front
Leica TCS SP8 Quick Start Guide Leica TCS SP8 System Overview Start-Up Procedure 1. Turn on the CTR Control Box, Fluorescent Light for the microscope stand. 2. Turn on the Scanner Power (1) on the front
Point Spread Function. Confocal Laser Scanning Microscopy. Confocal Aperture. Optical aberrations. Alternative Scanning Microscopy
 Bi177 Lecture 5 Adding the Third Dimension Wide-field Imaging Point Spread Function Deconvolution Confocal Laser Scanning Microscopy Confocal Aperture Optical aberrations Alternative Scanning Microscopy
Bi177 Lecture 5 Adding the Third Dimension Wide-field Imaging Point Spread Function Deconvolution Confocal Laser Scanning Microscopy Confocal Aperture Optical aberrations Alternative Scanning Microscopy
Wide-Field TCSPC FLIM with bh SPC-150 N TCSPC System and Photek FGN Detector
 Wide-Field TCSPC FLIM with bh SPC-150 N TCSPC System and Photek FGN 392-1000 Detector Abstract: We present a wide-field TCSPC FLIM system consisting of a position-sensitive MCP PMT of the delay-line type,
Wide-Field TCSPC FLIM with bh SPC-150 N TCSPC System and Photek FGN 392-1000 Detector Abstract: We present a wide-field TCSPC FLIM system consisting of a position-sensitive MCP PMT of the delay-line type,
The Zeiss AiryScan System, Confocal Four.
 The Zeiss AiryScan System, Confocal Four. Overview. The Zeiss AiryScan module is a segmented, radially stacked GaASP detector and collector system designed to subsample the airy disk of a point emission
The Zeiss AiryScan System, Confocal Four. Overview. The Zeiss AiryScan module is a segmented, radially stacked GaASP detector and collector system designed to subsample the airy disk of a point emission
2013 LMIC Imaging Workshop. Sidney L. Shaw Technical Director. - Light and the Image - Detectors - Signal and Noise
 2013 LMIC Imaging Workshop Sidney L. Shaw Technical Director - Light and the Image - Detectors - Signal and Noise The Anatomy of a Digital Image Representative Intensities Specimen: (molecular distribution)
2013 LMIC Imaging Workshop Sidney L. Shaw Technical Director - Light and the Image - Detectors - Signal and Noise The Anatomy of a Digital Image Representative Intensities Specimen: (molecular distribution)
Lecture 20: Optical Tools for MEMS Imaging
 MECH 466 Microelectromechanical Systems University of Victoria Dept. of Mechanical Engineering Lecture 20: Optical Tools for MEMS Imaging 1 Overview Optical Microscopes Video Microscopes Scanning Electron
MECH 466 Microelectromechanical Systems University of Victoria Dept. of Mechanical Engineering Lecture 20: Optical Tools for MEMS Imaging 1 Overview Optical Microscopes Video Microscopes Scanning Electron
Zeiss 780 Training Notes
 Zeiss 780 Training Notes Turn on Main Switch, System PC and Components Switches 780 Start up sequence Do you need the argon laser (458, 488, 514 nm lines)? Yes Turn on the laser s main power switch and
Zeiss 780 Training Notes Turn on Main Switch, System PC and Components Switches 780 Start up sequence Do you need the argon laser (458, 488, 514 nm lines)? Yes Turn on the laser s main power switch and
Introduction. Lighting
 &855(17 )8785(75(1'6,10$&+,1(9,6,21 5HVHDUFK6FLHQWLVW0DWV&DUOLQ 2SWLFDO0HDVXUHPHQW6\VWHPVDQG'DWD$QDO\VLV 6,17()(OHFWURQLFV &\EHUQHWLFV %R[%OLQGHUQ2VOR125:$< (PDLO0DWV&DUOLQ#HF\VLQWHIQR http://www.sintef.no/ecy/7210/
&855(17 )8785(75(1'6,10$&+,1(9,6,21 5HVHDUFK6FLHQWLVW0DWV&DUOLQ 2SWLFDO0HDVXUHPHQW6\VWHPVDQG'DWD$QDO\VLV 6,17()(OHFWURQLFV &\EHUQHWLFV %R[%OLQGHUQ2VOR125:$< (PDLO0DWV&DUOLQ#HF\VLQWHIQR http://www.sintef.no/ecy/7210/
Electron-Bombarded CMOS
 New Megapixel Single Photon Position Sensitive HPD: Electron-Bombarded CMOS University of Lyon / CNRS-IN2P3 in collaboration with J. Baudot, E. Chabanat, P. Depasse, W. Dulinski, N. Estre, M. Winter N56:
New Megapixel Single Photon Position Sensitive HPD: Electron-Bombarded CMOS University of Lyon / CNRS-IN2P3 in collaboration with J. Baudot, E. Chabanat, P. Depasse, W. Dulinski, N. Estre, M. Winter N56:
Image acquisition. In both cases, the digital sensing element is one of the following: Line array Area array. Single sensor
 Image acquisition Digital images are acquired by direct digital acquisition (digital still/video cameras), or scanning material acquired as analog signals (slides, photographs, etc.). In both cases, the
Image acquisition Digital images are acquired by direct digital acquisition (digital still/video cameras), or scanning material acquired as analog signals (slides, photographs, etc.). In both cases, the
Akinori Mitani and Geoff Weiner BGGN 266 Spring 2013 Non-linear optics final report. Introduction and Background
 Akinori Mitani and Geoff Weiner BGGN 266 Spring 2013 Non-linear optics final report Introduction and Background Two-photon microscopy is a type of fluorescence microscopy using two-photon excitation. It
Akinori Mitani and Geoff Weiner BGGN 266 Spring 2013 Non-linear optics final report Introduction and Background Two-photon microscopy is a type of fluorescence microscopy using two-photon excitation. It
DCS-120. Confocal Scanning FLIM Systems. Based on bh s Multidimensional Megapixel FLIM Technology
 Based on bh s Multidimensional Megapixel FLIM Technology Complete Laser Scanning FLIM Microscopes FLIM Upgrades for Existing Conventional Microscopes Multidimensional TCSPC technique High throughput dual-channel
Based on bh s Multidimensional Megapixel FLIM Technology Complete Laser Scanning FLIM Microscopes FLIM Upgrades for Existing Conventional Microscopes Multidimensional TCSPC technique High throughput dual-channel
Case Study: Custom CCD for X-ray Free Electron Laser Experiment
 Introduction The first XFEL (X-ray Free Electron Laser) experiments are being constructed around the world. These facilities produce femto-second long bursts of the most intense coherent X-rays ever to
Introduction The first XFEL (X-ray Free Electron Laser) experiments are being constructed around the world. These facilities produce femto-second long bursts of the most intense coherent X-rays ever to
Technology Note ZEISS LSM 880 with Airyscan
 Technology Note ZEISS LSM 880 with Airyscan Introducing the Fast Acquisition Mode ZEISS LSM 880 with Airyscan Introducing the Fast Acquisition Mode Author: Dr. Annette Bergter Carl Zeiss Microscopy GmbH,
Technology Note ZEISS LSM 880 with Airyscan Introducing the Fast Acquisition Mode ZEISS LSM 880 with Airyscan Introducing the Fast Acquisition Mode Author: Dr. Annette Bergter Carl Zeiss Microscopy GmbH,
Opterra. Multipoint Scanning Confocal Microscope. Innovation with Integrity. Cell-Friendly, High-Speed, High-Resolution Imaging
 Opterra Multipoint Scanning Confocal Microscope Cell-Friendly, High-Speed, High-Resolution Imaging Innovation with Integrity Fluorescence Microscopy Opterra Multipoint Scanning Confocal Microscope Superior
Opterra Multipoint Scanning Confocal Microscope Cell-Friendly, High-Speed, High-Resolution Imaging Innovation with Integrity Fluorescence Microscopy Opterra Multipoint Scanning Confocal Microscope Superior
3D light microscopy techniques
 3D light microscopy techniques The image of a point is a 3D feature In-focus image Out-of-focus image The image of a point is not a point Point Spread Function (PSF) 1D imaging 1 1 2! NA = 0.5! NA 2D imaging
3D light microscopy techniques The image of a point is a 3D feature In-focus image Out-of-focus image The image of a point is not a point Point Spread Function (PSF) 1D imaging 1 1 2! NA = 0.5! NA 2D imaging
product overview pco.edge family the most versatile scmos camera portfolio on the market pioneer in scmos image sensor technology
 product overview family the most versatile scmos camera portfolio on the market pioneer in scmos image sensor technology scmos knowledge base scmos General Information PCO scmos cameras are a breakthrough
product overview family the most versatile scmos camera portfolio on the market pioneer in scmos image sensor technology scmos knowledge base scmos General Information PCO scmos cameras are a breakthrough
TRAINING MANUAL. Multiphoton Microscopy LSM 510 META-NLO
 TRAINING MANUAL Multiphoton Microscopy LSM 510 META-NLO September 2010 Multiphoton Microscopy Training Manual Multiphoton microscopy is only available on the LSM 510 META-NLO system. This system is equipped
TRAINING MANUAL Multiphoton Microscopy LSM 510 META-NLO September 2010 Multiphoton Microscopy Training Manual Multiphoton microscopy is only available on the LSM 510 META-NLO system. This system is equipped
Confocal, hyperspectral, spinning disk
 Confocal, hyperspectral, spinning disk Administrative HW 6 due on Fri Midterm on Wed Covers everything since previous midterm 8.5 x 11 sheet allowed, 1 side Guest lecture by Joe Dragavon on Mon 10/30 Last
Confocal, hyperspectral, spinning disk Administrative HW 6 due on Fri Midterm on Wed Covers everything since previous midterm 8.5 x 11 sheet allowed, 1 side Guest lecture by Joe Dragavon on Mon 10/30 Last
Spark Spectral Sensor Offers Advantages
 04/08/2015 Spark Spectral Sensor Offers Advantages Spark is a small spectral sensor from Ocean Optics that bridges the spectral measurement gap between filter-based devices such as RGB color sensors and
04/08/2015 Spark Spectral Sensor Offers Advantages Spark is a small spectral sensor from Ocean Optics that bridges the spectral measurement gap between filter-based devices such as RGB color sensors and
Last class. This class. CCDs Fancy CCDs. Camera specs scmos
 CCDs and scmos Last class CCDs Fancy CCDs This class Camera specs scmos Fancy CCD cameras: -Back thinned -> higher QE -Unexposed chip -> frame transfer -Electron multiplying -> higher SNR -Fancy ADC ->
CCDs and scmos Last class CCDs Fancy CCDs This class Camera specs scmos Fancy CCD cameras: -Back thinned -> higher QE -Unexposed chip -> frame transfer -Electron multiplying -> higher SNR -Fancy ADC ->
Purchasing a Back-illuminated scmos for Microscopy? Seven Reasons To Choose Sona
 Purchasing a Back-illuminated scmos for Microscopy? Seven Reasons To Choose Sona Dr. Colin Coates, Andor July 2018 Technical Note Purchasing a Back-illuminated scmos for Microscopy: 7 Reasons to Choose
Purchasing a Back-illuminated scmos for Microscopy? Seven Reasons To Choose Sona Dr. Colin Coates, Andor July 2018 Technical Note Purchasing a Back-illuminated scmos for Microscopy: 7 Reasons to Choose
A 4 Megapixel camera with 6.5μm pixels, Prime BSI captures highly. event goes undetected.
 PRODUCT DATASHEET Prime BSI SCIENTIFIC CMOS CAMERA Can a camera single-handedly differentiate your product against competitors? With the Prime BSI, the answer is a resounding yes. Instrument builders no
PRODUCT DATASHEET Prime BSI SCIENTIFIC CMOS CAMERA Can a camera single-handedly differentiate your product against competitors? With the Prime BSI, the answer is a resounding yes. Instrument builders no
Digital Camera Technologies for Scientific Bio-Imaging. Part 2: Sampling and Signal
 Digital Camera Technologies for Scientific Bio-Imaging. Part 2: Sampling and Signal Yashvinder Sabharwal, 1 James Joubert 2 and Deepak Sharma 2 1. Solexis Advisors LLC, Austin, TX, USA 2. Photometrics
Digital Camera Technologies for Scientific Bio-Imaging. Part 2: Sampling and Signal Yashvinder Sabharwal, 1 James Joubert 2 and Deepak Sharma 2 1. Solexis Advisors LLC, Austin, TX, USA 2. Photometrics
pco.edge 4.2 LT 0.8 electrons 2048 x 2048 pixel 40 fps : 1 > 70 % pco. low noise high resolution high speed high dynamic range
 edge 4.2 LT scientific CMOS camera high resolution 2048 x 2048 pixel low noise 0.8 electrons USB 3.0 small form factor high dynamic range 36 000 : 1 high speed 40 fps high quantum efficiency > 70 % edge
edge 4.2 LT scientific CMOS camera high resolution 2048 x 2048 pixel low noise 0.8 electrons USB 3.0 small form factor high dynamic range 36 000 : 1 high speed 40 fps high quantum efficiency > 70 % edge
Precision-tracking of individual particles By Fluorescence Photo activation Localization Microscopy(FPALM) Presented by Aung K.
 Precision-tracking of individual particles By Fluorescence Photo activation Localization Microscopy(FPALM) Presented by Aung K. Soe This FPALM research was done by Assistant Professor Sam Hess, physics
Precision-tracking of individual particles By Fluorescence Photo activation Localization Microscopy(FPALM) Presented by Aung K. Soe This FPALM research was done by Assistant Professor Sam Hess, physics
Confocal Microscopy. Kristin Jensen
 Confocal Microscopy Kristin Jensen 17.11.05 References Cell Biological Applications of Confocal Microscopy, Brian Matsumoto, chapter 1 Studying protein dynamics in living cells,, Jennifer Lippincott-Schwartz
Confocal Microscopy Kristin Jensen 17.11.05 References Cell Biological Applications of Confocal Microscopy, Brian Matsumoto, chapter 1 Studying protein dynamics in living cells,, Jennifer Lippincott-Schwartz
Things to check before start-up.
 Byeong Cha Page 1 11/24/2009 Manual for Leica SP2 Confocal Microscope Enter you name, the date, the time, and the account number in the user log book. Things to check before start-up. Make sure that your
Byeong Cha Page 1 11/24/2009 Manual for Leica SP2 Confocal Microscope Enter you name, the date, the time, and the account number in the user log book. Things to check before start-up. Make sure that your
Introduction to light microscopy
 Center for Microscopy and Image Anaylsis Introduction to light microscopy (an overview) Microscopy with light Components of a light microscope 1. Light source 2. Objective 3. Sample or specimen holder
Center for Microscopy and Image Anaylsis Introduction to light microscopy (an overview) Microscopy with light Components of a light microscope 1. Light source 2. Objective 3. Sample or specimen holder
Εισαγωγική στην Οπτική Απεικόνιση
 Εισαγωγική στην Οπτική Απεικόνιση Δημήτριος Τζεράνης, Ph.D. Εμβιομηχανική και Βιοϊατρική Τεχνολογία Τμήμα Μηχανολόγων Μηχανικών Ε.Μ.Π. Χειμερινό Εξάμηνο 2015 Light: A type of EM Radiation EM radiation:
Εισαγωγική στην Οπτική Απεικόνιση Δημήτριος Τζεράνης, Ph.D. Εμβιομηχανική και Βιοϊατρική Τεχνολογία Τμήμα Μηχανολόγων Μηχανικών Ε.Μ.Π. Χειμερινό Εξάμηνο 2015 Light: A type of EM Radiation EM radiation:
Microscopic Structures
 Microscopic Structures Image Analysis Metal, 3D Image (Red-Green) The microscopic methods range from dark field / bright field microscopy through polarisation- and inverse microscopy to techniques like
Microscopic Structures Image Analysis Metal, 3D Image (Red-Green) The microscopic methods range from dark field / bright field microscopy through polarisation- and inverse microscopy to techniques like
Training Guide for Carl Zeiss LSM 5 LIVE Confocal Microscope
 Training Guide for Carl Zeiss LSM 5 LIVE Confocal Microscope AIM 4.2 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Verify that main power switches on the
Training Guide for Carl Zeiss LSM 5 LIVE Confocal Microscope AIM 4.2 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Verify that main power switches on the
CHARGE-COUPLED DEVICE (CCD)
 CHARGE-COUPLED DEVICE (CCD) Definition A charge-coupled device (CCD) is an analog shift register, enabling analog signals, usually light, manipulation - for example, conversion into a digital value that
CHARGE-COUPLED DEVICE (CCD) Definition A charge-coupled device (CCD) is an analog shift register, enabling analog signals, usually light, manipulation - for example, conversion into a digital value that
Grant No. DE-FG-05-85ER Progress Report One. R. H. Atalla. The Institute of Paper Chemistry. April, Prepared for
 DOE DE-FG-05-85ER75212 THE INSTITUTE OF PAPER CHEMISTRY, APPLETON, WISCONSIN TIME RESOLVED RAMAN MICROPROBE SYSTEM Grant No. DE-FG-05-85ER75212 Progress Report One R. H. Atalla The Institute of Paper Chemistry
DOE DE-FG-05-85ER75212 THE INSTITUTE OF PAPER CHEMISTRY, APPLETON, WISCONSIN TIME RESOLVED RAMAN MICROPROBE SYSTEM Grant No. DE-FG-05-85ER75212 Progress Report One R. H. Atalla The Institute of Paper Chemistry
pco.edge electrons 2048 x 1536 pixel 50 fps :1 > 60 % pco. low noise high resolution high speed high dynamic range
 edge 3.1 scientific CMOS camera high resolution 2048 x 1536 pixel low noise 1.1 electrons global shutter USB 3.0 small form factor high dynamic range 27 000:1 high speed 50 fps high quantum efficiency
edge 3.1 scientific CMOS camera high resolution 2048 x 1536 pixel low noise 1.1 electrons global shutter USB 3.0 small form factor high dynamic range 27 000:1 high speed 50 fps high quantum efficiency
Flatness of Dichroic Beamsplitters Affects Focus and Image Quality
 Flatness of Dichroic Beamsplitters Affects Focus and Image Quality Flatness of Dichroic Beamsplitters Affects Focus and Image Quality 1. Introduction Even though fluorescence microscopy has become a routine
Flatness of Dichroic Beamsplitters Affects Focus and Image Quality Flatness of Dichroic Beamsplitters Affects Focus and Image Quality 1. Introduction Even though fluorescence microscopy has become a routine
3D light microscopy techniques
 3D light microscopy techniques The image of a point is a 3D feature In-focus image Out-of-focus image The image of a point is not a point Point Spread Function (PSF) 1D imaging 2D imaging 3D imaging Resolution
3D light microscopy techniques The image of a point is a 3D feature In-focus image Out-of-focus image The image of a point is not a point Point Spread Function (PSF) 1D imaging 2D imaging 3D imaging Resolution
CFIM MICROSCOPY COURSE PROGRAMME PRINCIPLES OF MICROSCOPY CONFOCAL AND FLUORESCENCE MICROSCOPY
 CFIM MICROSCOPY COURSE PROGRAMME PRINCIPLES OF MICROSCOPY 11.01.16-15.01.2016 CONFOCAL AND FLUORESCENCE MICROSCOPY 25.01.16-29.01.2016 PhD Course - University of Copenhagen Department of Biomedical Sciences
CFIM MICROSCOPY COURSE PROGRAMME PRINCIPLES OF MICROSCOPY 11.01.16-15.01.2016 CONFOCAL AND FLUORESCENCE MICROSCOPY 25.01.16-29.01.2016 PhD Course - University of Copenhagen Department of Biomedical Sciences
EE 392B: Course Introduction
 EE 392B Course Introduction About EE392B Goals Topics Schedule Prerequisites Course Overview Digital Imaging System Image Sensor Architectures Nonidealities and Performance Measures Color Imaging Recent
EE 392B Course Introduction About EE392B Goals Topics Schedule Prerequisites Course Overview Digital Imaging System Image Sensor Architectures Nonidealities and Performance Measures Color Imaging Recent
Bi Imaging. Multicolor Imaging: The Important Question of Co-Localization. Anna Smallcombe Bio-Rad Laboratories, Hemel Hempstead, UK
 Multicolor Imaging: The Important Question of Co-Localization Anna Smallcombe Bio-Rad Laboratories, Hemel Hempstead, UK The use of specific fluorescent probes, combined with confocal or multiphoton microscopy
Multicolor Imaging: The Important Question of Co-Localization Anna Smallcombe Bio-Rad Laboratories, Hemel Hempstead, UK The use of specific fluorescent probes, combined with confocal or multiphoton microscopy
pco.edge 4.2 LT 0.8 electrons 2048 x 2048 pixel 40 fps up to :1 up to 82 % pco. low noise high resolution high speed high dynamic range
 edge 4.2 LT scientific CMOS camera high resolution 2048 x 2048 pixel low noise 0.8 electrons USB 3.0 small form factor high dynamic range up to 37 500:1 high speed 40 fps high quantum efficiency up to
edge 4.2 LT scientific CMOS camera high resolution 2048 x 2048 pixel low noise 0.8 electrons USB 3.0 small form factor high dynamic range up to 37 500:1 high speed 40 fps high quantum efficiency up to
Imaging Beyond the Basics: Optimizing Settings on the Leica SP8 Confocal
 Imaging Beyond the Basics: Optimizing Settings on the Leica SP8 Confocal Todays Goal: Introduce some additional functionalities of the Leica SP8 confocal HyD vs. PMT detectors Dye Assistant Scanning By
Imaging Beyond the Basics: Optimizing Settings on the Leica SP8 Confocal Todays Goal: Introduce some additional functionalities of the Leica SP8 confocal HyD vs. PMT detectors Dye Assistant Scanning By
Maria Smedh, Centre for Cellular Imaging. Maria Smedh, Centre for Cellular Imaging
 Nonlinear microscopy I: Two-photon fluorescence microscopy Multiphoton Microscopy What is multiphoton imaging? Applications Different imaging modes Advantages/disadvantages Scattering of light in thick
Nonlinear microscopy I: Two-photon fluorescence microscopy Multiphoton Microscopy What is multiphoton imaging? Applications Different imaging modes Advantages/disadvantages Scattering of light in thick
Megapixel FLIM with bh TCSPC Modules
 Megapixel FLIM with bh TCSPC Modules The New SPCM 64-bit Software Abstract: Becker & Hickl have recently introduced version 9.60 of their SPCM TCSPC data acquisition software. SPCM version 9.60 not only
Megapixel FLIM with bh TCSPC Modules The New SPCM 64-bit Software Abstract: Becker & Hickl have recently introduced version 9.60 of their SPCM TCSPC data acquisition software. SPCM version 9.60 not only
DU-897 (back illuminated)
 IMAGING Andor s ixon EM + DU-897 back illuminated EMCCD has single photon detection capability without an image intensifier, combined with greater than 90% QE of a back-illuminated sensor. Containing a
IMAGING Andor s ixon EM + DU-897 back illuminated EMCCD has single photon detection capability without an image intensifier, combined with greater than 90% QE of a back-illuminated sensor. Containing a
Light Microscopy. Upon completion of this lecture, the student should be able to:
 Light Light microscopy is based on the interaction of light and tissue components and can be used to study tissue features. Upon completion of this lecture, the student should be able to: 1- Explain the
Light Light microscopy is based on the interaction of light and tissue components and can be used to study tissue features. Upon completion of this lecture, the student should be able to: 1- Explain the
User manual for Olympus SD-OSR spinning disk confocal microscope
 User manual for Olympus SD-OSR spinning disk confocal microscope Ved Prakash, PhD. Research imaging specialist Imaging & histology core University of Texas, Dallas ved.prakash@utdallas.edu Once you open
User manual for Olympus SD-OSR spinning disk confocal microscope Ved Prakash, PhD. Research imaging specialist Imaging & histology core University of Texas, Dallas ved.prakash@utdallas.edu Once you open
Microscopy from Carl Zeiss
 Microscopy from Carl Zeiss Contents Page Contents... 1 Introduction... 1 Starting the System... 2 Introduction to ZEN Efficient Navigation... 5 Setting up the microscope... 10 Configuring the beam path
Microscopy from Carl Zeiss Contents Page Contents... 1 Introduction... 1 Starting the System... 2 Introduction to ZEN Efficient Navigation... 5 Setting up the microscope... 10 Configuring the beam path
Operation Guide for the Leica SP2 Confocal Microscope Bio-Imaging Facility Hunter College October 2009
 Operation Guide for the Leica SP2 Confocal Microscope Bio-Imaging Facility Hunter College October 2009 Introduction of Fluoresence Confocal Microscopy The first confocal microscope was invented by Princeton
Operation Guide for the Leica SP2 Confocal Microscope Bio-Imaging Facility Hunter College October 2009 Introduction of Fluoresence Confocal Microscopy The first confocal microscope was invented by Princeton
Zeiss Axiovert 135 Fluorescence Microscope Quick Guide / Operations Manual (v. 1.0 February 09)
 University of Chicago Integrated Light Microscopy Core Dr. Vytas Bindokas, Director http://digital.bsd.uchicago.edu By: Christine Labno, Assistant Director Room: AB-129 Phone: 4-9040 Zeiss Axiovert 135
University of Chicago Integrated Light Microscopy Core Dr. Vytas Bindokas, Director http://digital.bsd.uchicago.edu By: Christine Labno, Assistant Director Room: AB-129 Phone: 4-9040 Zeiss Axiovert 135
Important performance parameters when considering lasers for holographic applications
 Important performance parameters when considering lasers for holographic applications E.K. Illy*, H. Karlsson & G. Elgcrona. Cobolt AB, a part of HÜBNER Photonics, Vretenvägen 13, 17154, Stockholm, Sweden.
Important performance parameters when considering lasers for holographic applications E.K. Illy*, H. Karlsson & G. Elgcrona. Cobolt AB, a part of HÜBNER Photonics, Vretenvägen 13, 17154, Stockholm, Sweden.
Working Simultaneously. The Next Level of TIRF Microscopy. cell^tirf Illuminator Motorized Total Internal Reflection Fluorescence
 cell^tirf Illuminator Motorized Total Internal Reflection Fluorescence Four individually aligned illumination beams for simultaneous multi-color TIRF imaging Working Simultaneously The Next Level of TIRF
cell^tirf Illuminator Motorized Total Internal Reflection Fluorescence Four individually aligned illumination beams for simultaneous multi-color TIRF imaging Working Simultaneously The Next Level of TIRF
Electron-Multiplying (EM) Gain 2006, 2007 QImaging. All rights reserved.
 D IGITAL IMAGING made easy TECHNICAL NOTE Electron-Multiplying (EM) Gain 26, 27 QImaging. All rights reserved. In order to gain a clearer understanding of biological processes at the single-molecule level,
D IGITAL IMAGING made easy TECHNICAL NOTE Electron-Multiplying (EM) Gain 26, 27 QImaging. All rights reserved. In order to gain a clearer understanding of biological processes at the single-molecule level,
1 Co Localization and Working flow with the lsm700
 1 Co Localization and Working flow with the lsm700 Samples -1 slide = mousse intestine, Dapi / Ki 67 with Cy3/ BrDU with alexa 488. -1 slide = mousse intestine, Dapi / Ki 67 with Cy3/ no BrDU (but with
1 Co Localization and Working flow with the lsm700 Samples -1 slide = mousse intestine, Dapi / Ki 67 with Cy3/ BrDU with alexa 488. -1 slide = mousse intestine, Dapi / Ki 67 with Cy3/ no BrDU (but with
Practical Flatness Tech Note
 Practical Flatness Tech Note Understanding Laser Dichroic Performance BrightLine laser dichroic beamsplitters set a new standard for super-resolution microscopy with λ/10 flatness per inch, P-V. We ll
Practical Flatness Tech Note Understanding Laser Dichroic Performance BrightLine laser dichroic beamsplitters set a new standard for super-resolution microscopy with λ/10 flatness per inch, P-V. We ll
Zeiss LSM 880 Protocol
 Zeiss LSM 880 Protocol 1) System Startup Please note put sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for unused time.
Zeiss LSM 880 Protocol 1) System Startup Please note put sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for unused time.
Applying Automated Optical Inspection Ben Dawson, DALSA Coreco Inc., ipd Group (987)
 Applying Automated Optical Inspection Ben Dawson, DALSA Coreco Inc., ipd Group bdawson@goipd.com (987) 670-2050 Introduction Automated Optical Inspection (AOI) uses lighting, cameras, and vision computers
Applying Automated Optical Inspection Ben Dawson, DALSA Coreco Inc., ipd Group bdawson@goipd.com (987) 670-2050 Introduction Automated Optical Inspection (AOI) uses lighting, cameras, and vision computers
GenePix Application Note
 GenePix Application Note Determining the Signal-to-Noise Ratio and Optimal Photomultiplier gain setting in the GenePix 4000B Siobhan Pickett, M.S., Sean Carriedo, Ph.D. and Chang Wang, Ph.D. Axon Instruments,
GenePix Application Note Determining the Signal-to-Noise Ratio and Optimal Photomultiplier gain setting in the GenePix 4000B Siobhan Pickett, M.S., Sean Carriedo, Ph.D. and Chang Wang, Ph.D. Axon Instruments,
Zeiss LSM 780 Protocol
 Zeiss LSM 780 Protocol 1) System Startup F Please note the sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for unused time.
Zeiss LSM 780 Protocol 1) System Startup F Please note the sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for unused time.
A new Infra-Red Camera for COAST. Richard Neill - PhD student Supervisor: Dr John Young
 A new Infra-Red Camera for COAST Richard Neill - PhD student Supervisor: Dr John Young The Cambridge Optical Aperture-Synthesis Telescope: COAST is a
A new Infra-Red Camera for COAST Richard Neill - PhD student Supervisor: Dr John Young The Cambridge Optical Aperture-Synthesis Telescope: COAST is a
Control of Noise and Background in Scientific CMOS Technology
 Control of Noise and Background in Scientific CMOS Technology Introduction Scientific CMOS (Complementary metal oxide semiconductor) camera technology has enabled advancement in many areas of microscopy
Control of Noise and Background in Scientific CMOS Technology Introduction Scientific CMOS (Complementary metal oxide semiconductor) camera technology has enabled advancement in many areas of microscopy
Lecture 23 MNS 102: Techniques for Materials and Nano Sciences
 Lecture 23 MNS 102: Techniques for Materials and Nano Sciences Reference: #1 C. R. Brundle, C. A. Evans, S. Wilson, "Encyclopedia of Materials Characterization", Butterworth-Heinemann, Toronto (1992),
Lecture 23 MNS 102: Techniques for Materials and Nano Sciences Reference: #1 C. R. Brundle, C. A. Evans, S. Wilson, "Encyclopedia of Materials Characterization", Butterworth-Heinemann, Toronto (1992),
Training Guide for Leica SP8 Confocal/Multiphoton Microscope
 Training Guide for Leica SP8 Confocal/Multiphoton Microscope LAS AF v3.3 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Turn ON power switch for epifluorescence
Training Guide for Leica SP8 Confocal/Multiphoton Microscope LAS AF v3.3 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Turn ON power switch for epifluorescence
Fundamentals of Light Microscopy II: Fluorescence, Deconvolution, Confocal, Multiphoton, Spectral microscopy. Integrated Microscopy Course
 Fundamentals of Light Microscopy II: Fluorescence, Deconvolution, Confocal, Multiphoton, Spectral microscopy Integrated Microscopy Course Review Lecture 1: Microscopy Basics Light train Kohler illumination*
Fundamentals of Light Microscopy II: Fluorescence, Deconvolution, Confocal, Multiphoton, Spectral microscopy Integrated Microscopy Course Review Lecture 1: Microscopy Basics Light train Kohler illumination*
Locating Molecules Using GSD Technology Project Folders: Organization of Experiment Files...1
 .....................................1 1 Project Folders: Organization of Experiment Files.................................1 2 Steps........................................................................2
.....................................1 1 Project Folders: Organization of Experiment Files.................................1 2 Steps........................................................................2
Introduction to light microscopy
 Center for Microscopy and Image Anaylsis Introduction to light Imaging with light / Overview of techniques Urs Ziegler ziegler@zmb.uzh.ch Light interacting with matter Absorbtion Refraction Diffraction
Center for Microscopy and Image Anaylsis Introduction to light Imaging with light / Overview of techniques Urs Ziegler ziegler@zmb.uzh.ch Light interacting with matter Absorbtion Refraction Diffraction
Leica TCS SP8 Quick Start Guide
 Leica TCS SP8 Quick Start Guide Leica TCS SP8 System Overview Start-Up Procedure 1. Turn on the CTR Control Box, EL6000 fluorescent light source for the microscope stand. 2. Turn on the Scanner Power
Leica TCS SP8 Quick Start Guide Leica TCS SP8 System Overview Start-Up Procedure 1. Turn on the CTR Control Box, EL6000 fluorescent light source for the microscope stand. 2. Turn on the Scanner Power
TCSPC at Wavelengths from 900 nm to 1700 nm
 TCSPC at Wavelengths from 900 nm to 1700 nm We describe picosecond time-resolved optical signal recording in the spectral range from 900 nm to 1700 nm. The system consists of an id Quantique id220 InGaAs
TCSPC at Wavelengths from 900 nm to 1700 nm We describe picosecond time-resolved optical signal recording in the spectral range from 900 nm to 1700 nm. The system consists of an id Quantique id220 InGaAs
High-resolution, low light-dose lightsheet microscope LATTICE LIGHTSHEET
 LATTICE LIGHTSHEET High-resolution, low light-dose lightsheet microscope First developed by Nobel Laureate Dr. Eric Betzig, the 3i Lattice LightSheet microscope is capable of imaging biological systems
LATTICE LIGHTSHEET High-resolution, low light-dose lightsheet microscope First developed by Nobel Laureate Dr. Eric Betzig, the 3i Lattice LightSheet microscope is capable of imaging biological systems
Pixel shift in fluorescence microscopy
 Pixel shift in fluorescence microscopy 1. Introduction Multicolor imaging in fluorescence microscopy is typically performed by sequentially acquiring images of different colors. An overlay of these images
Pixel shift in fluorescence microscopy 1. Introduction Multicolor imaging in fluorescence microscopy is typically performed by sequentially acquiring images of different colors. An overlay of these images
Components of Optical Instruments
 Components of Optical Instruments General Design of Optical Instruments Sources of Radiation Wavelength Selectors (Filters, Monochromators, Interferometers) Sample Containers Radiation Transducers (Detectors)
Components of Optical Instruments General Design of Optical Instruments Sources of Radiation Wavelength Selectors (Filters, Monochromators, Interferometers) Sample Containers Radiation Transducers (Detectors)
Bio 407. Applied microscopy. Introduction into light microscopy. José María Mateos. Center for Microscopy and Image Analysis
 Center for Microscopy and Image Analysis Bio 407 Applied Introduction into light José María Mateos Fundamentals of light Compound microscope Microscope composed of an objective and an additional lens (eyepiece,
Center for Microscopy and Image Analysis Bio 407 Applied Introduction into light José María Mateos Fundamentals of light Compound microscope Microscope composed of an objective and an additional lens (eyepiece,
Image Capture TOTALLAB
 1 Introduction In order for image analysis to be performed on a gel or Western blot, it must first be converted into digital data. Good image capture is critical to guarantee optimal performance of automated
1 Introduction In order for image analysis to be performed on a gel or Western blot, it must first be converted into digital data. Good image capture is critical to guarantee optimal performance of automated
Nikon. King s College London. Imaging Centre. N-SIM guide NIKON IMAGING KING S COLLEGE LONDON
 N-SIM guide NIKON IMAGING CENTRE @ KING S COLLEGE LONDON Starting-up / Shut-down The NSIM hardware is calibrated after system warm-up occurs. It is recommended that you turn-on the system for at least
N-SIM guide NIKON IMAGING CENTRE @ KING S COLLEGE LONDON Starting-up / Shut-down The NSIM hardware is calibrated after system warm-up occurs. It is recommended that you turn-on the system for at least
Using interlaced restart reset cameras. Documentation Addendum
 Using interlaced restart reset cameras on Domino Iota, Alpha 2 and Delta boards December 27, 2005 WARNING EURESYS S.A. shall retain all rights, title and interest in the hardware or the software, documentation
Using interlaced restart reset cameras on Domino Iota, Alpha 2 and Delta boards December 27, 2005 WARNING EURESYS S.A. shall retain all rights, title and interest in the hardware or the software, documentation
A new Photon Counting Detector: Intensified CMOS- APS
 A new Photon Counting Detector: Intensified CMOS- APS M. Belluso 1, G. Bonanno 1, A. Calì 1, A. Carbone 3, R. Cosentino 1, A. Modica 4, S. Scuderi 1, C. Timpanaro 1, M. Uslenghi 2 1-I.N.A.F.-Osservatorio
A new Photon Counting Detector: Intensified CMOS- APS M. Belluso 1, G. Bonanno 1, A. Calì 1, A. Carbone 3, R. Cosentino 1, A. Modica 4, S. Scuderi 1, C. Timpanaro 1, M. Uslenghi 2 1-I.N.A.F.-Osservatorio
More fancy SPIM, Even fancier SPIM
 More fancy SPIM, Even fancier SPIM Last class Light sheet microscopy Fancy SPIM (ispim, dspim, etc ) This class Multi camera SPIM SIM SPIM Bessels d x,y = λ em 2 NA d z = 2 NA λ ex + n(1 cosθ λ em 1 IsoView
More fancy SPIM, Even fancier SPIM Last class Light sheet microscopy Fancy SPIM (ispim, dspim, etc ) This class Multi camera SPIM SIM SPIM Bessels d x,y = λ em 2 NA d z = 2 NA λ ex + n(1 cosθ λ em 1 IsoView
Training Guide for Carl Zeiss LSM 880 with AiryScan FAST
 Training Guide for Carl Zeiss LSM 880 with AiryScan FAST ZEN 2.3 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2018) Power ON Routine 1 2 Turn ON Main Switch from the remote control
Training Guide for Carl Zeiss LSM 880 with AiryScan FAST ZEN 2.3 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2018) Power ON Routine 1 2 Turn ON Main Switch from the remote control
Nanonics Systems are the Only SPMs that Allow for On-line Integration with Standard MicroRaman Geometries
 Nanonics Systems are the Only SPMs that Allow for On-line Integration with Standard MicroRaman Geometries 2002 Photonics Circle of Excellence Award PLC Ltd, England, a premier provider of Raman microspectral
Nanonics Systems are the Only SPMs that Allow for On-line Integration with Standard MicroRaman Geometries 2002 Photonics Circle of Excellence Award PLC Ltd, England, a premier provider of Raman microspectral
pco.edge 4.2 LT 0.8 electrons 2048 x 2048 pixel 40 fps :1 > 70 % pco. low noise high resolution high speed high dynamic range
 edge 4.2 LT scientific CMOS camera high resolution 2048 x 2048 pixel low noise 0.8 electrons USB 3.0 small form factor high dynamic range 37 500:1 high speed 40 fps high quantum efficiency > 70 % edge
edge 4.2 LT scientific CMOS camera high resolution 2048 x 2048 pixel low noise 0.8 electrons USB 3.0 small form factor high dynamic range 37 500:1 high speed 40 fps high quantum efficiency > 70 % edge
