Standard Operating Procedure Bruker Quest Diffractometer with copper wavelength X-ray microsource
|
|
|
- Myles Rolf Potter
- 6 years ago
- Views:
Transcription
1 Standard Operating Procedure Bruker Quest Diffractometer with copper wavelength X-ray microsource X-ray Crystallography Laboratory Purdue Department of Chemistry The following is a guide for collecting data and solving structures using the Purdue Bruker Quest single crystal diffractometer with a copper wavelength X-ray microsource. It is intended as a walk-through user guide geared especially towards novice users, but also tries to cover more advanced features of the software where they are important for the collection of normal simple small molecule structural data. A basic knowledge of the fundamentals of diffraction and crystallography is expected. This manual is based on an instrument and software produced around the year Most of the manual s content also applies to newer as well as older instrument makes. For the novice user, changes between different generation instruments are mostly limited to slightly different program layouts and color schemes. The general procedures described in this manual still apply. For a more in-depth description of the features of a CCD or CMOS diffractometer, the reader should refer to the manuals and technique guides on specific topics by the manufacturer of the type of instrumentation you are using. The gold standard for a more in depth guide towards the use of Shelxtl for the refinement of single crystal structures is Peter Müller s book Crystal Structure Refinement. Every crystallography lab should have at least one copy. Among the many programs commonly used for crystal structure solution and refinement we recommend the Bruker Shelxtl package (including XPREP, XS and XM), George Sheldrick s refinement program Shelxl2017, the graphical interface Shelxle by Hübschle, Dittrich and Sheldrick, and Platon by Anthony Spek. 1
2 Table of Contents Instrument Overview... 3 Adjusting the Temperature... 4 Starting the System... 5 Unloading the Previous Sample... 6 Selecting and Mounting of Sample... 7 Crystal Mounting and Centering... 9 Crystal and Compound Description Unit Cell Determination Data Collection Data Integration Scaling and Absorption Correction Transferring the Data Setting up Data for XPREP XPREP The Initial INS File Solving Structures using XS and XM Structure Refinement
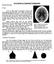
3 Instrument Overview The Bruker Quest instrument with a copper microsource consists of: The diffractometer including the X-ray enclosure, detector, mircosource IµSCu X-ray tube, multilayer optics for monochromatization and collimator, video microscope, kappa (4-axis) goniometer, electronic controls, power supply and other miscellaneous pieces of equipment essential for the operation of the instrument. An Oxford Cryosystems 800 variable temperature unit (pump, controllers and liquid nitrogen tanks located outside the enclosure) and an Oxford Cryosystems AD51 dry air unit. The instrument computer: This computer runs the Measurement Server, the Bruker Instrument Service (BIS), Diffrac.Maintenance and Apex3. Figure 1, Inside of the Quest X-ray enclosure with microsource X-ray tube, Detector, Variable Temp Unit and Goniometer. Between X-ray tube and detector are the Optics (Multilayer Optics for monochromatization, Collimator), the Video Microscope (in back, partially hidden), and the Beam Stop. On the Goniometer is mounted the Goniometer Head holding the crystal specimen. 3
4 Having a promising sample of crystals, it is best to start in the following order: Adjusting the Temperature All but very high melting samples (inorganics, ceramics, etc) should be measured at lower temperature to avoid extensive thermal motion of the atom cores, to obtain higher angle diffraction data and to minimize radiation damage to the sample. The Oxford Cryosystems variable temperature unit is a stand-alone unit, operated through its own controllers. It can be also controlled via Apex3 or BIS. The sample is embedded in a stream of cold nitrogen gas supplied by a low pressure liquid nitrogen dewar. To avoid buildup of ice, the sample is insulated from ambient humidity by an outer layer of warm dry air supplied by an Oxford Cryosystems AD51 dry air unit (located outside the lab in a facilities closet). The Oxford Cryosystems variable temperature unit is able to achieve temperatures between 375 K ( C) down to ca. 85 K ( C). The recommended temperature for low temperature data collections is 100 K in winter, and 150 K in summer (due to higher humidity levels that can cause icing around the sample mount). Make sure, the tank is properly connected and full enough for the planned experiment. The Oxford Cryostream N2 tank (silver dewar behind the diffractometer) is automatically refilled from the larger low pressure liquid nitrogen tank (between the two diffractometers). Check that the Liquid Level Controller (the smaller of the two controllers, Fig. 2) is set to <AUTO> for automatic refills. The fill level displayed on the controller should be between 20 and 80%. When it is below 20% (despite the controller set to AUTO) the low pressure N2 tank is empty and needs to be refilled. 4
5 Figure 2, The external controller (bottom) and the circulation pump (top) of the Oxford Cryosystems variable temperature unit. Check that no data collection is running (e.g., look at the BIS interface, check for Frames left or Time left ). If a running data collection does not need to continue abort the data collection (see below), then make changes to the VT unit. Check that the dry air unit providing the shield gas flow is switched on. Check on the display of the Cryostream Controller (larger of the two controllers) if the variable temperature unit might be already running. If the unit is running check if the temperature is appropriate for your experiment. If not, use the <Run Experiment> interface of Apex3 to set the desired temperature (see below for using Apex3). If the unit is switched off it needs to be started. Press the main single button on the front of the controller. Follow the on screen prompts to switch on the unit. Use the <Run Experiment> interface of Apex3 to set the desired temperature (see below for using Apex3). Starting the System Start BIS (Bruker Instrumentation System) if it is not already running. This will also start the measurement server if it is not active already. 5
6 Figure 3, The BIS window Unloading the Previous Sample Check if a data collection is still running (e.g., look at the BIS interface, check for Frames left or Time left ). If a data collection is active but can be aborted, then stop this data collection first. Navigate to the active copy of Apex3 and click the STOP symbol in the Apex3 interface. Minimize any sessions of Apex3 that might be open. Start a new session of Apex3. No login is required. Connect to BIS (<Instrument>, then <Connect>). Via the <Sample> dropdown menu, either <Open > an existing sample (continuation of old data collection) or Start a <New > Sample Fill in the project name. Avoid using special characters (including dashes and periods) or overly long project names. Under <Set Up>, go to <Center Crystal> Click on <Center> in the lower right of the interface. The angles will drive to a position convenient for mounting a magnetic snap-on mount. 6
7 Press the <door open> button on the front face of the diffractometer enclosure (to the right of the front door, below the light button). Doors need to be closed to drive any mechanical parts other than the phi (spindle) axis of the goniometer. Carefully dismount the magnetic snap-on mount from the previous experiment. Tilt it backwards until the magnet comes loose, then take it off. Figure 4, Goniometer head with magnetic snap on mount and adjustment screw Selecting and Mounting of Sample Crystals can be mounted on glass rods, inside glass capillaries, on Nylon loops, or using mesh mounts. The Purdue X-ray lab uses Mitegen micromesh mounts for most samples and data collections. Two types of micromesh mounts for small (<0.1 mm) and medium and large ( mm) crystals are stocked in the lab (Fig 5). Crystals collected at low temperature are mounted on the micromesh with the help of a trace of mineral oil, Fomblin (fluorinated mineral oil) or polybutene oil (very viscous oil to protect extremely oxygen sensitive samples) and flash 7
8 frozen in the cold stream. Crystals collected at room or elevated temperature might need to be fixed to the mesh with a trace of glue to avoid crystal movement during measurement. Figure 5, Tips of Mitegen Micromesh mounts as seen through a microscope (Left: small tipped mount with 15 µm openings for crystals 0.1 mm and smaller. Right: large mesh mount with 25 µm openings. Mesh is 0.4 mm across). For typical crystal selection and mounting, place a drop of mineral or Fomblin oil on a glass slide under a microscope. Place several representative crystals in the oil. Inspect the crystals and select a suitable candidate. Crystals on the Quest instrument with copper microsource should ideally be not larger than mm in any direction (the size of the X-ray beam is 0.1 mm). The minimum size depends on the diffraction intensity of the crystal. Larger crystals, especially if they are heavily absorbing, are usually more suitable for the Quest instrument with molybdenum sealed tube X-ray source (the size of its X-ray beam is 0.6 mm). Data collections are much faster using molybdenum radiation as long as the achievable diffraction is sufficiently intense. The higher intensity microscource and the intrinsically better diffraction with copper radiation is ideally suited for small weakly diffracting crystals that are unsuitable for molybdenum radiation. Only those samples and organic samples for which absolute configuration is required should be run on the copper wavelength instrument. For highly absorbing samples limit the size of the crystal (make sure the minimum transmission will be above 10-20%). If no single specimen with suitable dimensions can be found, use a sharp razor blade to cut off a single piece from a larger crystal or cluster. Remove all smaller pieces and dirt from the crystal (the micromesh mounts can be used to brush off loose pieces from the crystal). 8
9 Figure 6, Crystals with well-developed faces on a microscope slide Scoop up a crystal with the mircomesh mount and place it on the inside of the concave face of the mount in the center of the mesh. Remove as much excess oil as possible. (If a crystal needs to be glued to the mount make sure that both crystal and mount are dry without any traces of oil. Dip the mesh carefully in a small amount of glue and pick up the crystal without touching the glass slide). Crystal Mounting and Centering At this point you should have a session of Apex3 running. Press the <door open> button on the front face of the diffractometer enclosure (to the right of the front door, below the light button). Carefully mount the magnetic snap on mount onto the goniometer. Check the position of the mount by eye (is it aligned with the collimator and the beam stop?) In Apex3, click <Center Crystal> in the <Set Up> submenue. Click the blue-green play button at the top of the window to open the live video stream feed. Is the crystal visible on the video screen? If you cannot see the crystal, its position is further off than usual and needs to be adjusted by eye on the goniometer head before the live video feed can be used. 9
10 Figure 7, Crystal Centering Window and live video feed with mounted sample and green measuring line. There are three pins on the goniometer head: for up-down, right-left, and forward-backward. Use the bold end of the adjustment screw driver to turn the pins to position the crystal in the center of the cross hair of the video feed. When the crystal is centered in the cross hair, click the large <Spin Phi 90> button (in the <Center Crystal> window of Apex3) to spin the crystal around the phi axis by 90. Repeat the centering procedure using the adjustment screws as before. Repeat the process (<Spin Phi 90> followed by centering of the crystal in the cross hair of the video feed) until the crystal stays positioned in the center of the cross hair in all positions. If the cross hair seems to be slightly off try to center the crystal so that it rotates in place. For oddly shaped crystals, try to center the mass center of the crystal. For long needles, try to align them along the axis of the mounting pin. Measure the dimensions of the crystal: Switch on the measuring tool (double sided arrow icon on top of <Center Crystal> window, Fig. 7). Click on one side of the crystal, hold the left mouse key and drag to the other side. The length of the green line dragged is given in the 10
11 bar below the video screen ( vector length, value in µm). Write down the value and repeat for the other two directions using <Spin Phi 90>. Crystal and Compound Description Under <Setup>, go to <Describe> Fill in all values that apply. In the compound line, it is best to give the full name of the person that provided the sample. Crystal dimensions need to be entered in mm (video screen values are in µm!). Figure 8, Crystal and compound description 11
12 Unit Cell Determination Under <Evaluate>, click on <Determine Unit Cell>. Two procedures are available, <Automatic Mode> or <Manual Mode>. Figure 9, Starting Window of the Unit Cell determination We encourage to use the manual procedure. To do so click <Collect Data>, this will open the Unit Cell Data Collection Window. Choose an exposure time (default is 10 seconds) and click <Collect> frames will be collected which will be displayed in the frame window. The first sweep will be at low angle, with the beam stop visible at the center (Figure 10). The second and third run (Figure 11) will be at high angle. Make sure that your specimen diffracts to sufficiently high angle. Use the resolution arc tool (Figure 11) to check the resolution limit. Only samples that show at least weak diffraction above ca. 0.9 Å (with ten second exposures) are likely to meet the IUCr requirements for publication. 12
13 Figure 10, Unit Cell window with the first (low angle) sweep running. Figure 11, Unit Cell window with one of the high angle sweeps running and resolution arc tool. 13
14 When finished proceed to <Harvest Spots>, select an I/sigma(I) cutoff value for the diffraction spots to be used (see the frame to the left, spots that will be used are green circled). To obtain a reasonable suggestion for the exposure time use the default value of Click on <Harvest>. Figure 12, Unit Cell Harvesting Window Proceed to <Index>, use all default values and click on <Index>. 14
15 Figure 13, Unit Cell Indexing Result Window Check the unit cells obtained. The positions of the diffraction spots should agree with the predicted positions (white or blue circles). The hkl histogram (on the right) should have values around 90% or higher in the 0.1 line. If neither of the two is the case a nonmerohedral twin might be present, or the crystal is not single. If this is the case, export the data as a p4p file using <Sample>, then <Export> and use the program Cell Now to determine the cell (see the Purdue Twinning Manual for details). If one of the two unit cells looks reasonable, select it and proceed to <Refine>. Use all defaults and click on <Refine>, then <Accept>. 15
16 Figure 14, Unit Cell Refinement Window Proceed to <Bravais>. A list of possible Bravais lattices will be displayed with the software's choice highlighted in green. 16
17 Figure 15, Bravais Result Window The correct choice should have an FOM (figure of merit) value significantly higher than the others. If several solutions have similarly high FOM values, the one with the highest symmetry is likely to be correct. If you are not sure (e.g. when a high symmetry solution has a high FOM value, but significantly lower than a lower symmetry solution) select the lower symmetry solution or your data set as set up in <Data Collection Strategy> might be incomplete. Proceed to <Refine>, click <Refine>, then <Accept>. Please note the resolution prediction at the bottom of the window This finishes the Unit Cell Determination. 17
18 Data Collection Click < Calculate Strategy> under <Collect>. Under Step 1., change the resolution to d = 0.82 Å or smaller. The worst acceptable value, by IUCr standard, is 0.83 Å. The best resolution that can be set on this instrument is 0.78 Å. Collecting to 0.78 rather than 0.82 significantly increases the data collection time and is only recommended for samples that do indeed intensely diffract all the way to the detector edge. Click <Apply> if you made any changes. Figure 16, Strategy Starting Window Under Step 2., click <Determine Strategy>. In the pop-up window, it is usually save to use the default values. Click <OK>, 18
19 Figure 17, Determine Strategy popup window Under Step 3., click <Select Scan Parameters>. A popup window will emerge with suggested values for frame angle and frame time. Figure 18, Strategy Window with Scan Parameters sub-window The values can usually be accepted as they are. 19
20 Under <Collect> click on <Experiment> Click <Append Strategy> to add the runs from the Strategy calculation. On this instrument, usually no Fast Scans are required. Instead, edit the exposure times for the low angle runs (runs with values < 30 degrees in the 2Theta column) to one half or one quarter of the other exposure times. For medium angles (30 60 degrees, use 2/3 rd of the suggested exposure time. This avoids overexposure of the detector for the more intense low and medium angle diffraction spots. The long exposure as estimated in strategy should be retained for high angle runs (> 60 degrees in 2Theta). To preliminarily solve your structure usually each one low angle and one high or medium angle run are needed on this instrument. If the first low angle run is scheduled by strategy rather late into the measurement then it might make sense to move it up in the order of runs. Click on the run you would like to move so that it is highlighted in dark blue. Move the mouse cursor over the number to the left of the line and right click. Use <cut> to remove the line. Highlight the first line, right mouse click on the number to the left again and use <paste> to move the run into first place. 20
21 Figure 19, Experiment setup window during editing of low angle exposure time Click <Execute> to start the data collection. The instrument will now collect your dataset. Open <Show Status> from the Instrument drop down menue. This will open the <Instrument Status> window where you can check variables and completion time. You can also check many of these items via the BIS interface. 21
22 Figure 20, Data Collection Window with active data collection and BIS Window in foreground Users are encouraged to check the progress of their data collection and to test-solve and refine the data while the data collection is still running to check that the data quality is good enough to proceed, that the structure is indeed of interest, that the unit cell is correct, and if data might be already complete. It is highly advisable to not stop a data collection until you are sure the data are complete enough for a meaningful absorption correction and to pass checkcif (i.e. data should be integrated, scaled, solved, refined, the refinement quality checked, the completeness should be checked in the cif file, and preliminarily checked using checkcif prior to stopping the data collection!!). Once a crystal is taken off the instrument a data collection cannot be resumed! 22
23 Data Integration When the data collection is complete (or when enough data are collected for an initial structure solution and refinement attempt) open, <Reduce Data>, then <Integrate Images> Change the resolution limit to 0.83 Å or lower Click on <Find Runs>, check the runs you would like to integrate (leave out the Matrix runs). Figure 21, Integration Start Window with <Select Runs> Window open Click on <Start Integration>. This will start the integration. Wait for it to finish, this might take several minutes. 23
24 Figure 22, Integration Window with active Integration running Spot Shape Correlation should show values around or above 80% and be mostly even. Values of 40% or lower are statistically meaningless. Pixel Errors should be on average between ±0.2. Average I/sigma(I) intensities should be above at least 3 (good datasets have values larger than 20). Shape profiles should be round to slightly ellipsoid. Double spots, large pixel errors and erratic uneven lines are possible signs for twinning. If you suspect a crystal to be split or twinned, consult the Purdue Twinning Manual. If you encounter problems with data completeness: Open the _0m._ls file and inspect the table at the beginning of this file. Inspect especially the values for Spots exceeding frame queue size and Spots left in Write-Behind Cache. If these are unusually large (a substantial fraction the total data), then click <Integration Options> and change the values for Profile XYX Half-Widths from 4 x 4 x 4 to 9 x 9 x 9. Repeat the integration. If you used very narrow frame widths you might also want to increase the number for Active Image Queue Half-Widths (e.g. from 7 to 15, maximum is 32). 24
25 Scaling and Absorption Correction Under <Reduce Data> Click on <Scale> Figure 23, Absorption Correction and Error Model (<Scale>) Starting Window The merged batch raw file from the integration is loaded automatically. If you did not integrate all runs at once you need to load individual raw files. Click the yellow browse symbol, click on any raw file you want to load, then click <OK>. Uncheck raw files you would like to not use. Check the Laue Group and Point Group. Keep Multi-scan as the absorption correction method. It is sufficient for > 99% of all samples. For heavy absorbers, adjust the number of Mu*r (mu is the absorption coefficient in mm -1, r the average radius of the crystal in mm). Proceed by clicking <Next>. 25
26 Figure 24, Absorption Correction window (second Scale Window) before refinement The refinement window will open (Fig. 24). An average redundancy of around 5.0 is usually sufficient for weak to medium absorbing samples. For oddly shaped and heavily absorbing samples a higher redundancy is recommended. Click <Refine>, wait for the refinement to finish and inspect the results (Fig. 25). 26
27 Figure 25, Absorption Correction window (second Scale Window) after refinement Click <Next>. Check the number of rejected reflections. If this number is extremely high, in conjunction with higher than expected data R values, then there is a possibility that the selected Laue group is too high in symmetry ( metric pseudosymmetry ). The number of rejected reflections can be reduced (assuming that the Laue group is correct!) by increasing the value for < I-<I> /su ratio for rejection> (default is 4.0). If you do so click <Error Model> to update the outlier rejection. 27
28 Figure 26, Error Model Window (third Scale Window) Click <Finish>, then <Exit> to finalize scaling and absorption correction. You have now finished the data collection and absorption correction. To allow other users to use the instrument you should move your data to a different computer at this point. Transferring the Data Move the data via a USB flash drive or online to the data workup computer or your personal computer. For the refinement, you will need the *.hkl and the *.p4p files (located in the work folder of your project) e.g. 04mz02a_0m.hkl 04mz02a_0m.p4p For publication purposes you will also need the *.abs and the *._ls files: e.g. 04mz02am.abs or 04mz02a_0m.abs (A copy of what you did in SCALE, SADABS, or TWINABS, contains the ratio of Tmin/Tmax) 28
29 04mz02am_0m._ls (A copy of the last lines of the integration procedure, contains parameters of unit cell refinement (2THETA min, 2THETA max and the number of reflections used), crystal colour and shape, crystal dimensions) If you used Cell Now to obtain a unit cell, you will also need to copy the *._cn file Setting up Data for XPREP On your computer save the files from above into a new file folder. Making a backup of your files is strongly recommended! (with older operating systems you need to uncheck the read only flag, by highlighting your files plus right mouse click, go into properties and uncheck.) Open the SHELXTL program. Select <PROJECT> and <New>. Find the appropriate file and open it. Give it a project name e.g. 04mz02a_0m, then <open>. Figure 3, Shelxtl Program and Project Manager Window with New Project Window open 29
30 XPREP Select <XPREP> on the toolbar. In the next steps, the computer will make suggestions that can usually be accepted (i.e. for high quality data). Figure 4, XPREP Window, initial lattice centering selection 30
31 - Select the suggested lattice type. - Choose [H] to search for higher metric symmetry. - Choose offered choice [A] for the Laue group (e.g. monoclinic). Figure 5, XPREP Window, initial lattice type selection - Select [S] to determine or input space group. - Select [S] again to determine space group. - Select the suggested Laue group (e.g. [M] for monoclinic) 31
32 - Select the suggested lattice centering (e.g. [P] for primitive, [C] for C-centered, etc) Figure 30, XPREP Window, new lattice centering selection - Select the suggested option for the space group e.g. P2(1)/c or C222(1) (If several solutions are offered, take the one with the lowest CFOM value. If that does not work out later on, try the next best in the list). 32
33 Figure 31, XPREP Window, space group selection - Select [D] to read, modify or merge datasets - Select [S] to display the intensity statistics - Select [A] to merge all equivalent reflections (including Friedel opposites). This does not actually merge the reflections (which is not recommended to do), but only displays the data and statistics as if they would have been merged. 33
34 The intensity statistics will be displayed. Figure 32, XPREP Window, intensity statistics Have a look at the Completeness, Redundancy, Rmerge and Rsigma (Rmerge is the same as Rint in Shelxl and Sadabs). The completeness should be ideally close to 100 % down to a resolution of d = 0.82 Å. Rsigma and Rmerge should be ideally below 10 % down to d = 0.82 Å. Hit the Enter key - Select [E] to exit to the main menu, then [C] to define the unit cell contents. - If no unit cell contents are given type the most likely molecular formula (element symbols and numbers only). - If the given formula seems wrong, select [F] for new formula, and type the most likely formula. 34
35 Figure 33, XPREP Window, formula definition - If necessary, select [R] to change the radiation (Mo radn; λ = ; Cu radn; λ = ) - If necessary, select [Z] to change the number of (symmetrically independent) molecules Z per unit cell. - Select [E] to exit to the main menu. Note: If you had been using SADABS for the generation of your *.hkl file, no absorption correction has to be applied. If you still need to apply absorption correction, this can be done here by choosing [A] (not covered here). - Select [F] to setup the new hkl file. If you have changed the unit cell or its orientation in XPREP, the program will force you to choose a new name for the hkl file to avoid overwriting and losing the original data (change it back to your old filename after closing XPREP, or start a new project with the new hkl filename). - Select [Y] at the prompt to generate an.ins file. 35
36 Figure 34, XPREP Window, ins and hkl file setup - Select [Q] at the prompt 36
37 The Initial INS File The initial *.ins file written in XPREP, together with the *.hkl file, contains all the information to get started solving and refining your crystal structure. The *.ins file can be opened and edited in any text editor. Open it via <Edit> and <Edit.ins> in the SHELXTL menu. The initial ins file may look like this: Figure 6, typical initial INS file The TITL line gives the initial name of the *.ins file and the space group from XPREP. The cell line gives the wavelength used, then the unit cell parameters a, b, c, α, β, γ. The ZERR line gives the Z value (number of molecules in the unit cell as given in XPREP) and the estimated standard deviations for a, b, c, α, β, γ. LATT gives the lattice type (see SHELXTL manual). The SYMM line(s) give the symmetry operators of the space group that create symmetry dependent atoms (these lines define the actual space group, not the TITL line). The SFAC line gives the atom types and defines the atom structure factors to be used by the program. The UNIT line gives the number of atoms per unit cell as listed in the SFAC line. The TEMP line gives the temperature in degrees Celsius. If needed, change the value to the actual temperature used in your experiment. The TREF line defines the type of structure solution method to be used (see below). 37
38 The HKLF 4 line defines the type of refinement to be used (HKLF 4 is the standard single crystal refinement for a not non-merohedrally twinned dataset). The END lines defines the end of the file. Select <file> and then <save>. Solving Structures using XS and XM To perform the refinement of your structure, you will first need an initial guess (a starting point). Using the *.ins file provided by XPREP, the programs XS or XM will provide you with this initial guess (other programs and methods can be used, such as DirDiff, charge flipping, etc). In the program XS there are two methods to choose from: Direct methods (indicated by the line TREF in the INS file), and the Patterson method (indicated by the line PATT in the ins file). Direct methods can be used for all datasets with better than atomic resolution. Patterson methods can only be used for structures with at least one heavy atom (i.e. sodium or heavier). Direct methods usually provide a more complete initial guess (more atoms already found), but they tend to fail more often than Patterson methods and they have occasionally problems to place inversion centers correctly. XM is utilizing direct methods originally written for macromolecular data, but can also be used for small molecule data. It is more likely to work where XS/TREF fails, but requires more computing resources. - To use XS, select <XS> on the toolbar and the computer begins to process data. The software tries in this step to find an initial guess for the atom positions. This can only be successful if the atoms listed in the formula are correct. The software will select the solution with the smallest value for CFOM (combined figure of merit) and the initial atom positions are written to the *.res file. The res file cannot be opened while XS is running. 38
39 Figure 7, the XS window - For good quality data the correct solution is normally falling out when continuing with Shelxle, Olex or Crystals graphical refinement interfaces. If this is not the case, you can change the settings for XS in the ins file. Go to <Edit> on the toolbar and select <edit.ins> Either, change TREF to TREF 2000 (or even higher) to run more iterations, or, for compounds with at least one atom heavier than sodium, use PATT instead of TREF. Select <file> and then <save> and run XS again. If XS fails using both PATT and TREF methods, try using <XM>. In the *.ins file, replace the TREF line by the following four lines: FIND 58 PLOP 77 MIND NTRY
40 Save the ins file and run Click <XM> in the in the SHELXTL menu. The software will run 1000 tries (or the number specified in the NTRY line) and selects the solution with the highest correlation coefficient, CC. The initial atom positions are written to the res file. This will take substantially longer than XS (a fast computer may be required), but solutions can be analyzed while XM is still running. If a valid solution is found, XM can be aborted. XM does not write all required lines to the *.res file. Before running the first refinement cycle, add the following lines: FMAP 2 L.S. 4 PLAN 20 Structure Refinement Open the result of the structure solution in a graphical interface such as Shexle, Olex or Crystals. See separate manuals for details of the refinement procedure, and for writing and validating the crystallographic information file. 40
Nature Protocols: doi: /nprot
 Supplementary Tutorial A total of nine examples illustrating different aspects of data processing referred to in the text are given here. Images for these examples can be downloaded from www.mrc- lmb.cam.ac.uk/harry/imosflm/examples.
Supplementary Tutorial A total of nine examples illustrating different aspects of data processing referred to in the text are given here. Images for these examples can be downloaded from www.mrc- lmb.cam.ac.uk/harry/imosflm/examples.
Data collection. Refinement. R[F 2 >2(F 2 )] = wr(f 2 ) = S = reflections 1118 parameters
![Data collection. Refinement. R[F 2 >2(F 2 )] = wr(f 2 ) = S = reflections 1118 parameters Data collection. Refinement. R[F 2 >2(F 2 )] = wr(f 2 ) = S = reflections 1118 parameters](/thumbs/90/102605898.jpg) organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Non-merohedrally twinned hexamethylenetetramine 4-nitrophenol water (1/2/1), triclinic modification Seik Weng
organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Non-merohedrally twinned hexamethylenetetramine 4-nitrophenol water (1/2/1), triclinic modification Seik Weng
Supplementary Figure 1
 Supplementary Figure 1 Technical overview drawing of the Roadrunner goniometer. The goniometer consists of three main components: an inline sample-viewing microscope, a high-precision scanning unit for
Supplementary Figure 1 Technical overview drawing of the Roadrunner goniometer. The goniometer consists of three main components: an inline sample-viewing microscope, a high-precision scanning unit for
Agilent Xcalibur and Gemini X-ray Diffractometers ENHANCED CHEMICAL CRYSTALLOGRAPHY SOLUTIONS
 Agilent Xcalibur and Gemini X-ray Diffractometers ENHANCED CHEMICAL CRYSTALLOGRAPHY SOLUTIONS AGILENT XCALIBUR AND GEMINI X-RAY DIFFRACTOMETERS ENHANCED CHEMICAL CRYSTALLOGRAPHY SOLUTIONS Agilent s Xcalibur
Agilent Xcalibur and Gemini X-ray Diffractometers ENHANCED CHEMICAL CRYSTALLOGRAPHY SOLUTIONS AGILENT XCALIBUR AND GEMINI X-RAY DIFFRACTOMETERS ENHANCED CHEMICAL CRYSTALLOGRAPHY SOLUTIONS Agilent s Xcalibur
Bruker D8 HRXRD. Collecting Reciprocal Space Maps using the LynxEye Position Sensitive Detector
 Bruker D8 HRXRD Collecting Reciprocal Space Maps using the LynxEye Position Sensitive Detector Scott A Speakman, Ph.D. MIT Center for Materials Science and Engineering For help in the X-ray Lab, contact
Bruker D8 HRXRD Collecting Reciprocal Space Maps using the LynxEye Position Sensitive Detector Scott A Speakman, Ph.D. MIT Center for Materials Science and Engineering For help in the X-ray Lab, contact
Basic Users Manual for Tecnai-F20 TEM
 Basic Users Manual for Tecnai-F20 TEM NB: This document contains my personal notes on the operating procedure of the Tecnai F20 and may be used as a rough guide for those new to the microscope. It may
Basic Users Manual for Tecnai-F20 TEM NB: This document contains my personal notes on the operating procedure of the Tecnai F20 and may be used as a rough guide for those new to the microscope. It may
Powder diffractometer operation instructions D8 Advance with a Cu Kα sealed tube and Lynxeye MARCH 30, 2016
 Powder diffractometer operation instructions D8 Advance with a Cu Kα sealed tube and Lynxeye MARCH 30, 2016 1 SAFETY. For radiation safety materials refer to http://www.ehs.wisc.edu/raddevices.htm. There
Powder diffractometer operation instructions D8 Advance with a Cu Kα sealed tube and Lynxeye MARCH 30, 2016 1 SAFETY. For radiation safety materials refer to http://www.ehs.wisc.edu/raddevices.htm. There
R-AXIS RAPID. X-ray Single Crystal Structure Analysis System. Product Information
 The Rigaku Journal Vol. 15/ number 2/ 1998 Product Information X-ray Single Crystal Structure Analysis System R-AXIS RAPID 1. Introduction X-ray single crystal structure analysis is known as the easiest
The Rigaku Journal Vol. 15/ number 2/ 1998 Product Information X-ray Single Crystal Structure Analysis System R-AXIS RAPID 1. Introduction X-ray single crystal structure analysis is known as the easiest
Standard Instructions for the Bruker D8 Advance Diffractometer, EPFL Valais Bragg Brentano and GID (Reflection)
 Standard Instructions for the Bruker D8 Advance Diffractometer, EPFL Valais Bragg Brentano and GID (Reflection) For any questions regarding the X-ray facility, contact: Pascal Schouwink pascal.schouwink@epfl.ch
Standard Instructions for the Bruker D8 Advance Diffractometer, EPFL Valais Bragg Brentano and GID (Reflection) For any questions regarding the X-ray facility, contact: Pascal Schouwink pascal.schouwink@epfl.ch
Experiment #12 X-Ray Diffraction Laboratory
 Physics 360/460 Experiment #12 X-Ray Diffraction Laboratory Introduction: To determine crystal lattice spacings, as well as identify unknown substances, a x-ray diffractometer is used to replace the traditional
Physics 360/460 Experiment #12 X-Ray Diffraction Laboratory Introduction: To determine crystal lattice spacings, as well as identify unknown substances, a x-ray diffractometer is used to replace the traditional
metal-organic compounds
 metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Monoclinic, C2=c a = 14.519 (3) Å b = 14.303 (3) Å c = 12.461 (3) Å = 101.94 (3) V = 2531.6 (9) Å 3 Z =4
metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Monoclinic, C2=c a = 14.519 (3) Å b = 14.303 (3) Å c = 12.461 (3) Å = 101.94 (3) V = 2531.6 (9) Å 3 Z =4
RENISHAW INVIA RAMAN SPECTROMETER
 STANDARD OPERATING PROCEDURE: RENISHAW INVIA RAMAN SPECTROMETER Purpose of this Instrument: The Renishaw invia Raman Spectrometer is an instrument used to analyze the Raman scattered light from samples
STANDARD OPERATING PROCEDURE: RENISHAW INVIA RAMAN SPECTROMETER Purpose of this Instrument: The Renishaw invia Raman Spectrometer is an instrument used to analyze the Raman scattered light from samples
Standard Operating Procedure for STOE STADI MP (IMSERC)
 Follow the general steps when computer has been restarted or last user has accidentally logged off. Please remember to: 1. Leave lab tables clean and tools/accessories organized 2. Return any unused masks
Follow the general steps when computer has been restarted or last user has accidentally logged off. Please remember to: 1. Leave lab tables clean and tools/accessories organized 2. Return any unused masks
FEI Tecnai G 2 F20 Operating Procedures
 FEI Tecnai G 2 F20 Operating Procedures 1. Startup (1) Sign-up in the microscope log-sheet. Please ensure you have written an account number for billing. (2) Log in to the computer: Login to your account
FEI Tecnai G 2 F20 Operating Procedures 1. Startup (1) Sign-up in the microscope log-sheet. Please ensure you have written an account number for billing. (2) Log in to the computer: Login to your account
= 0.74 mm 1 T = 100 K. Data collection. Refinement. R[F 2 >2(F 2 )] = wr(f 2 ) = S = reflections 423 parameters 1 restraint
![= 0.74 mm 1 T = 100 K. Data collection. Refinement. R[F 2 >2(F 2 )] = wr(f 2 ) = S = reflections 423 parameters 1 restraint = 0.74 mm 1 T = 100 K. Data collection. Refinement. R[F 2 >2(F 2 )] = wr(f 2 ) = S = reflections 423 parameters 1 restraint](/thumbs/94/121843619.jpg) organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 1-Cyclohexyl-2-(3-furyl)-1Hbenzimidazole-5-carboxylic acid Sergey Dibrov, Sanjay Dutta and Thomas Hermann* University
organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 1-Cyclohexyl-2-(3-furyl)-1Hbenzimidazole-5-carboxylic acid Sergey Dibrov, Sanjay Dutta and Thomas Hermann* University
CIF access. trans-(r,r)-2,2'-(cyclopenta-1,2-diyl)diphenyl Bis[(R)-O-methylmandelate] V. M. Lynch, R. Apodaca, J. K. Whitesell and M. I.
![CIF access. trans-(r,r)-2,2'-(cyclopenta-1,2-diyl)diphenyl Bis[(R)-O-methylmandelate] V. M. Lynch, R. Apodaca, J. K. Whitesell and M. I. CIF access. trans-(r,r)-2,2'-(cyclopenta-1,2-diyl)diphenyl Bis[(R)-O-methylmandelate] V. M. Lynch, R. Apodaca, J. K. Whitesell and M. I.](/thumbs/87/96678000.jpg) CIF access Acta Cryst. (1998). C54, IUC9800052 [ doi:10.1107/s0108270198099296 ] trans-(r,r)-2,2'-(cyclopenta-1,2-diyl)diphenyl Bis[(R)-O-methylmandelate] V. M. Lynch, R. Apodaca, J. K. Whitesell and M.
CIF access Acta Cryst. (1998). C54, IUC9800052 [ doi:10.1107/s0108270198099296 ] trans-(r,r)-2,2'-(cyclopenta-1,2-diyl)diphenyl Bis[(R)-O-methylmandelate] V. M. Lynch, R. Apodaca, J. K. Whitesell and M.
1. Preliminary sample preparation
 FEI Helios NanoLab 600 standard operating procedure Nicholas G. Rudawski ngr@ufl.edu (352) 392 3077 (office) (805) 252-4916 (cell) Last updated: 03/02/18 What this document provides: an overview of basic
FEI Helios NanoLab 600 standard operating procedure Nicholas G. Rudawski ngr@ufl.edu (352) 392 3077 (office) (805) 252-4916 (cell) Last updated: 03/02/18 What this document provides: an overview of basic
organic compounds Phenyl N-(4-fluorophenyl)carbamate o1036 Yang and Wang doi: /s Acta Cryst. (2009).
 organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Phenyl N-(4-fluorophenyl)carbamate Zhao Yang and Zhi-Xiang Wang* Department of Pharmaceutical Engineering, China
organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Phenyl N-(4-fluorophenyl)carbamate Zhao Yang and Zhi-Xiang Wang* Department of Pharmaceutical Engineering, China
CHAPTER1: QUICK START...3 CAMERA INSTALLATION... 3 SOFTWARE AND DRIVER INSTALLATION... 3 START TCAPTURE...4 TCAPTURE PARAMETER SETTINGS... 5 CHAPTER2:
 Image acquisition, managing and processing software TCapture Instruction Manual Key to the Instruction Manual TC is shortened name used for TCapture. Help Refer to [Help] >> [About TCapture] menu for software
Image acquisition, managing and processing software TCapture Instruction Manual Key to the Instruction Manual TC is shortened name used for TCapture. Help Refer to [Help] >> [About TCapture] menu for software
PANalytical X pert Pro Gazing Incidence X-ray Reflectivity User Manual (Version: )
 University of Minnesota College of Science and Engineering Characterization Facility PANalytical X pert Pro Gazing Incidence X-ray Reflectivity User Manual (Version: 2012.10.17) The following instructions
University of Minnesota College of Science and Engineering Characterization Facility PANalytical X pert Pro Gazing Incidence X-ray Reflectivity User Manual (Version: 2012.10.17) The following instructions
Recording EPR spectra using the Loop Gap Resonator (LGR)
 Recording EPR spectra using the Loop Gap Resonator (LGR) This protocol gives step-by-step instructions for recording EPR spectra of spin labeled proteins (Nitroxide label like MTSSL) using the LGR assuming
Recording EPR spectra using the Loop Gap Resonator (LGR) This protocol gives step-by-step instructions for recording EPR spectra of spin labeled proteins (Nitroxide label like MTSSL) using the LGR assuming
GXCapture 8.1 Instruction Manual
 GT Vision image acquisition, managing and processing software GXCapture 8.1 Instruction Manual Contents of the Instruction Manual GXC is the shortened name used for GXCapture Square brackets are used to
GT Vision image acquisition, managing and processing software GXCapture 8.1 Instruction Manual Contents of the Instruction Manual GXC is the shortened name used for GXCapture Square brackets are used to
Full-screen mode Popup controls. Overview of the microscope user interface, TEM User Interface and TIA on the left and EDS on the right
 Quick Guide to Operating FEI Titan Themis G2 200 (S)TEM: TEM mode Susheng Tan Nanoscale Fabrication and Characterization Facility, University of Pittsburgh Office: M104/B01 Benedum Hall, 412-383-5978,
Quick Guide to Operating FEI Titan Themis G2 200 (S)TEM: TEM mode Susheng Tan Nanoscale Fabrication and Characterization Facility, University of Pittsburgh Office: M104/B01 Benedum Hall, 412-383-5978,
Radial Polarization Converter With LC Driver USER MANUAL
 ARCoptix Radial Polarization Converter With LC Driver USER MANUAL Arcoptix S.A Ch. Trois-portes 18 2000 Neuchâtel Switzerland Mail: info@arcoptix.com Tel: ++41 32 731 04 66 Principle of the radial polarization
ARCoptix Radial Polarization Converter With LC Driver USER MANUAL Arcoptix S.A Ch. Trois-portes 18 2000 Neuchâtel Switzerland Mail: info@arcoptix.com Tel: ++41 32 731 04 66 Principle of the radial polarization
Procedures for Performing Cryoelectron Microscopy on the FEI Sphera Microscope
 Procedures for Performing Cryoelectron Microscopy on the FEI Sphera Microscope The procedures given below were written specifically for the FEI Tecnai G 2 Sphera microscope. Modifications will need to
Procedures for Performing Cryoelectron Microscopy on the FEI Sphera Microscope The procedures given below were written specifically for the FEI Tecnai G 2 Sphera microscope. Modifications will need to
Horiba LabRAM ARAMIS Raman Spectrometer Revision /28/2016 Page 1 of 11. Horiba Jobin-Yvon LabRAM Aramis - Raman Spectrometer
 Page 1 of 11 Horiba Jobin-Yvon LabRAM Aramis - Raman Spectrometer The Aramis Raman system is a software selectable multi-wavelength Raman system with mapping capabilities with a 400mm monochromator and
Page 1 of 11 Horiba Jobin-Yvon LabRAM Aramis - Raman Spectrometer The Aramis Raman system is a software selectable multi-wavelength Raman system with mapping capabilities with a 400mm monochromator and
Atomic and nuclear physics LD. Fine structure of the characteristic x-radiation of an iron anode. Physics
 Atomic and nuclear physics LD Physics X-ray physics Structure of x-ray spectra Leaflets P6.3.6.3 Fine structure of the characteristic x-radiation of an iron anode Objects of the experiment g Investigating
Atomic and nuclear physics LD Physics X-ray physics Structure of x-ray spectra Leaflets P6.3.6.3 Fine structure of the characteristic x-radiation of an iron anode Objects of the experiment g Investigating
MicroLab 500-series Getting Started
 MicroLab 500-series Getting Started 2 Contents CHAPTER 1: Getting Started Connecting the Hardware....6 Installing the USB driver......6 Installing the Software.....8 Starting a new Experiment...8 CHAPTER
MicroLab 500-series Getting Started 2 Contents CHAPTER 1: Getting Started Connecting the Hardware....6 Installing the USB driver......6 Installing the Software.....8 Starting a new Experiment...8 CHAPTER
Experimental. Crystal data. C 22 H 28 N 2 O 3 CH 4 O M r = Monoclinic, C2=c a = (4) Å b = (2) Å c = (5) Å = 96.
 organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 N 0 -(3,5-Di-tert-butyl-4-hydroxybenzylidene)-2-hydroxybenzohydrazide methanol solvate Wagee A. Yehye, Azhar Ariffin*
organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 N 0 -(3,5-Di-tert-butyl-4-hydroxybenzylidene)-2-hydroxybenzohydrazide methanol solvate Wagee A. Yehye, Azhar Ariffin*
PANalytical X pert Pro High Resolution Specular and Rocking Curve Scans User Manual (Version: )
 University of Minnesota College of Science and Engineering Characterization Facility PANalytical X pert Pro High Resolution Specular and Rocking Curve Scans User Manual (Version: 2012.10.17) The following
University of Minnesota College of Science and Engineering Characterization Facility PANalytical X pert Pro High Resolution Specular and Rocking Curve Scans User Manual (Version: 2012.10.17) The following
metal-organic compounds
 metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Bis[l-1,2-bis(1H-imidazol-1-ylmethyl)- benzene-j 2 N 3 :N 30 ]disilver(i) bis(4- amino-2,5-dichlorobenzenesulfonate)
metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Bis[l-1,2-bis(1H-imidazol-1-ylmethyl)- benzene-j 2 N 3 :N 30 ]disilver(i) bis(4- amino-2,5-dichlorobenzenesulfonate)
DISCO DICING SAW SOP. April 2014 INTRODUCTION
 DISCO DICING SAW SOP April 2014 INTRODUCTION The DISCO Dicing saw is an essential piece of equipment that allows cleanroom users to divide up their processed wafers into individual chips. The dicing saw
DISCO DICING SAW SOP April 2014 INTRODUCTION The DISCO Dicing saw is an essential piece of equipment that allows cleanroom users to divide up their processed wafers into individual chips. The dicing saw
Be aware that there is no universal notation for the various quantities.
 Fourier Optics v2.4 Ray tracing is limited in its ability to describe optics because it ignores the wave properties of light. Diffraction is needed to explain image spatial resolution and contrast and
Fourier Optics v2.4 Ray tracing is limited in its ability to describe optics because it ignores the wave properties of light. Diffraction is needed to explain image spatial resolution and contrast and
DATA COLLECTION WITH R-AXIS II, AN X-RAY DETECTING SYSTEM USING IMAGING PLATE
 THE RIGAKU JOURNAL VOL. 7 / NO. 2 / 1990 Technical Note DIFFRACTION DATA COLLECTION WITH R-AXIS II, AN X-RAY DETECTING SYSTEM USING IMAGING PLATE ATSUSHI SHIBATA R&D Division, Rigaku Corporation 1. Introduction
THE RIGAKU JOURNAL VOL. 7 / NO. 2 / 1990 Technical Note DIFFRACTION DATA COLLECTION WITH R-AXIS II, AN X-RAY DETECTING SYSTEM USING IMAGING PLATE ATSUSHI SHIBATA R&D Division, Rigaku Corporation 1. Introduction
UNIVERSITY OF WATERLOO Physics 360/460 Experiment #2 ATOMIC FORCE MICROSCOPY
 UNIVERSITY OF WATERLOO Physics 360/460 Experiment #2 ATOMIC FORCE MICROSCOPY References: http://virlab.virginia.edu/vl/home.htm (University of Virginia virtual lab. Click on the AFM link) An atomic force
UNIVERSITY OF WATERLOO Physics 360/460 Experiment #2 ATOMIC FORCE MICROSCOPY References: http://virlab.virginia.edu/vl/home.htm (University of Virginia virtual lab. Click on the AFM link) An atomic force
data reports Bis{2-[({[3-(dimethylazaniumyl)propyl]imino}- methyl)phenyl]sulfanido}nickel(ii) tetraphenylborate Structure description
![data reports Bis{2-[({[3-(dimethylazaniumyl)propyl]imino}- methyl)phenyl]sulfanido}nickel(ii) tetraphenylborate Structure description data reports Bis{2-[({[3-(dimethylazaniumyl)propyl]imino}- methyl)phenyl]sulfanido}nickel(ii) tetraphenylborate Structure description](/thumbs/95/125071125.jpg) ISSN 2414-3146 Bis{2-[({[3-(dimethylazaniumyl)propyl]imino}- methyl)phenyl]sulfanido}nickel(ii) tetraphenylborate Alexandra Meloccaro, Joshua R. Zimmerman and David M. Eichhorn* Received 25 June 2017 Accepted
ISSN 2414-3146 Bis{2-[({[3-(dimethylazaniumyl)propyl]imino}- methyl)phenyl]sulfanido}nickel(ii) tetraphenylborate Alexandra Meloccaro, Joshua R. Zimmerman and David M. Eichhorn* Received 25 June 2017 Accepted
LSM 780 Confocal Microscope Standard Operation Protocol
 LSM 780 Confocal Microscope Standard Operation Protocol Basic Operation Turning on the system 1. Sign on log sheet according to Actual start time 2. Check Compressed Air supply for the air table 3. Switch
LSM 780 Confocal Microscope Standard Operation Protocol Basic Operation Turning on the system 1. Sign on log sheet according to Actual start time 2. Check Compressed Air supply for the air table 3. Switch
AgilEye Manual Version 2.0 February 28, 2007
 AgilEye Manual Version 2.0 February 28, 2007 1717 Louisiana NE Suite 202 Albuquerque, NM 87110 (505) 268-4742 support@agiloptics.com 2 (505) 268-4742 v. 2.0 February 07, 2007 3 Introduction AgilEye Wavefront
AgilEye Manual Version 2.0 February 28, 2007 1717 Louisiana NE Suite 202 Albuquerque, NM 87110 (505) 268-4742 support@agiloptics.com 2 (505) 268-4742 v. 2.0 February 07, 2007 3 Introduction AgilEye Wavefront
metal-organic papers Hydridotetrakis(triphenylphosphito)cobalt(I) Comment Experimental
 metal-organic papers Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Hydridotetrakis(triphenylphosphito)cobalt(I) Jonathan D. Crane* and Nigel Young Department of Chemistry, University
metal-organic papers Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Hydridotetrakis(triphenylphosphito)cobalt(I) Jonathan D. Crane* and Nigel Young Department of Chemistry, University
Instructions for Tecnai a brief start up manual
 Instructions for Tecnai a brief start up manual Version 3.0, 8.12.2015 Manual of Tecnai 12 transmission electron microscope located at Aalto University's Nanomicroscopy Center. More information of Nanomicroscopy
Instructions for Tecnai a brief start up manual Version 3.0, 8.12.2015 Manual of Tecnai 12 transmission electron microscope located at Aalto University's Nanomicroscopy Center. More information of Nanomicroscopy
Figure 1 The Raith 150 TWO
 RAITH 150 TWO SOP Figure 1 The Raith 150 TWO LOCATION: Raith 150 TWO room, Lithography area, NanoFab PRIMARY TRAINER: SECONDARY TRAINER: 1. OVERVIEW The Raith 150 TWO is an ultra high resolution, low voltage
RAITH 150 TWO SOP Figure 1 The Raith 150 TWO LOCATION: Raith 150 TWO room, Lithography area, NanoFab PRIMARY TRAINER: SECONDARY TRAINER: 1. OVERVIEW The Raith 150 TWO is an ultra high resolution, low voltage
RAITH e-line OPERATING INSTRUCTIONS
 RAITH e-line OPERATING INSTRUCTIONS 1) LOADING A SAMPLE a. Start the system i. On the Column PC (Right side monitor [R]), select the SmartSEM icon to on the desktop to begin the column software. ii. On
RAITH e-line OPERATING INSTRUCTIONS 1) LOADING A SAMPLE a. Start the system i. On the Column PC (Right side monitor [R]), select the SmartSEM icon to on the desktop to begin the column software. ii. On
Experimental. Crystal data. C 19 H 18 O 2 M r = Orthorhombic, Pbca a = (3) Å b = (7) Å c = (5) Å.
 organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 3-Methyl-5-(3-phenoxyphenyl)cyclohex- 2-enone R. T. Sabapathy Mohan, a S. Kamatchi, a M. Subramanyam, b A. Thiruvalluvar
organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 3-Methyl-5-(3-phenoxyphenyl)cyclohex- 2-enone R. T. Sabapathy Mohan, a S. Kamatchi, a M. Subramanyam, b A. Thiruvalluvar
5-Chloro-2-(thiophen-2-yl)-1-(thiophen-2-ylmethyl)-1H- benzimidazole 6-Chloro-2-(thiophen-2-yl)-1-(thiophen-2- ylmethyl)-1h-benzimidazole (0.94/0.
 Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 5-Chloro-2-(thiophen-2-yl)-1-(thiophen-2-ylmethyl)-1H- benzimidazole 6-Chloro-2-(thiophen-2-yl)-1-(thiophen-2- ylmethyl)-1h-benzimidazole
Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 5-Chloro-2-(thiophen-2-yl)-1-(thiophen-2-ylmethyl)-1H- benzimidazole 6-Chloro-2-(thiophen-2-yl)-1-(thiophen-2- ylmethyl)-1h-benzimidazole
Leica DMi8A Quick Guide
 Leica DMi8A Quick Guide 1 Optical Microscope Quick Start Guide The following instructions are provided as a Quick Start Guide for powering up, running measurements, and shutting down Leica s DMi8A Inverted
Leica DMi8A Quick Guide 1 Optical Microscope Quick Start Guide The following instructions are provided as a Quick Start Guide for powering up, running measurements, and shutting down Leica s DMi8A Inverted
ENSC 470/894 Lab 3 Version 6.0 (Nov. 19, 2015)
 ENSC 470/894 Lab 3 Version 6.0 (Nov. 19, 2015) Purpose The purpose of the lab is (i) To measure the spot size and profile of the He-Ne laser beam and a laser pointer laser beam. (ii) To create a beam expander
ENSC 470/894 Lab 3 Version 6.0 (Nov. 19, 2015) Purpose The purpose of the lab is (i) To measure the spot size and profile of the He-Ne laser beam and a laser pointer laser beam. (ii) To create a beam expander
CrysAlis Pro : New Features in Agilent's User-Inspired X-ray Crystallography Software Platform
 CrysAlis Pro : New Features in Agilent's User-Inspired X-ray Crystallography Software Platform Mathias Meyer Agilent Technologies Poland Oliver Presly Agilent Technologies UK Crystallographic Software:
CrysAlis Pro : New Features in Agilent's User-Inspired X-ray Crystallography Software Platform Mathias Meyer Agilent Technologies Poland Oliver Presly Agilent Technologies UK Crystallographic Software:
Pixel Array Detectors: Counting and Integrating
 Pixel Array Detectors: Counting and Integrating Roger Durst, Bruker AXS October 13, 2016 1 The quest for a perfect detector There is, of course, no perfect detector All available detector technologies
Pixel Array Detectors: Counting and Integrating Roger Durst, Bruker AXS October 13, 2016 1 The quest for a perfect detector There is, of course, no perfect detector All available detector technologies
Operating the Hitachi 7100 Transmission Electron Microscope Electron Microscopy Core, University of Utah
 Operating the Hitachi 7100 Transmission Electron Microscope Electron Microscopy Core, University of Utah Follow the procedures below when you use the Hitachi 7100 TEM. Starting Session 1. Turn on the cold
Operating the Hitachi 7100 Transmission Electron Microscope Electron Microscopy Core, University of Utah Follow the procedures below when you use the Hitachi 7100 TEM. Starting Session 1. Turn on the cold
General Measurement (BB) Part
 General Measurement (BB) Part Contents Contents 1. How to set Part conditions...1 1.1 Setting conditions... 1 1.2 Setting measurement origin and oscillation/spin conditions... 7 General Measurement (BB)
General Measurement (BB) Part Contents Contents 1. How to set Part conditions...1 1.1 Setting conditions... 1 1.2 Setting measurement origin and oscillation/spin conditions... 7 General Measurement (BB)
2 How to operate the microscope/obtain an image
 Morgagni Operating Instructions 50079 010912 2-1 2 ow to operate the microscope/obtain an image 2.1 Starting the microscope 2.1.1 Starting the microscope with several manually-operated steps 1. Turn on
Morgagni Operating Instructions 50079 010912 2-1 2 ow to operate the microscope/obtain an image 2.1 Starting the microscope 2.1.1 Starting the microscope with several manually-operated steps 1. Turn on
Studuino Icon Programming Environment Guide
 Studuino Icon Programming Environment Guide Ver 0.9.6 4/17/2014 This manual introduces the Studuino Software environment. As the Studuino programming environment develops, these instructions may be edited
Studuino Icon Programming Environment Guide Ver 0.9.6 4/17/2014 This manual introduces the Studuino Software environment. As the Studuino programming environment develops, these instructions may be edited
LSM 710 Confocal Microscope Standard Operation Protocol
 LSM 710 Confocal Microscope Standard Operation Protocol Basic Operation Turning on the system 1. Switch on Main power switch 2. Switch on System / PC power button 3. Switch on Components power button 4.
LSM 710 Confocal Microscope Standard Operation Protocol Basic Operation Turning on the system 1. Switch on Main power switch 2. Switch on System / PC power button 3. Switch on Components power button 4.
Using the R-Axis Spider to run thin films
 Using the R-Axis Spider to run thin films In addition to loose powder samples, it is possible to run thin film samples on the R-Axis Spider. Use the zero background plate holder for your thin film sample.
Using the R-Axis Spider to run thin films In addition to loose powder samples, it is possible to run thin film samples on the R-Axis Spider. Use the zero background plate holder for your thin film sample.
KoPa Scanner. User's Manual A99. Ver 1.0. SHENZHEN OSTEC OPTO-ELECTRONIC TECHNOLOGY CO.,LTD.
 KoPa Scanner A99 User's Manual Ver 1.0 SHENZHEN OSTEC OPTO-ELECTRONIC TECHNOLOGY CO.,LTD. http://www.ostec.com.cn Content Chapter 1 Start... 1 1.1 Safety Warnings and Precautions... 1 1.2 Installation
KoPa Scanner A99 User's Manual Ver 1.0 SHENZHEN OSTEC OPTO-ELECTRONIC TECHNOLOGY CO.,LTD. http://www.ostec.com.cn Content Chapter 1 Start... 1 1.1 Safety Warnings and Precautions... 1 1.2 Installation
EKA Laboratory Muon Lifetime Experiment Instructions. October 2006
 EKA Laboratory Muon Lifetime Experiment Instructions October 2006 0 Lab setup and singles rate. When high-energy cosmic rays encounter the earth's atmosphere, they decay into a shower of elementary particles.
EKA Laboratory Muon Lifetime Experiment Instructions October 2006 0 Lab setup and singles rate. When high-energy cosmic rays encounter the earth's atmosphere, they decay into a shower of elementary particles.
Multiwire Real-Time Back-Reflection Laue Diffractometer
 Standard Operating Procedure for the Multiwire Real-Time Back-Reflection Laue Diffractometer Scott A Speakman, Ph.D Center for Materials Science and Engineering at MIT Speakman@mit.edu 617-253-6887 http://prism.mit.edu/xray
Standard Operating Procedure for the Multiwire Real-Time Back-Reflection Laue Diffractometer Scott A Speakman, Ph.D Center for Materials Science and Engineering at MIT Speakman@mit.edu 617-253-6887 http://prism.mit.edu/xray
Bruker D8 HRXRD Collecting X-Ray Reflectivity Data using the PathFinder Detector
 Bruker D8 HRXRD Collecting X-Ray Reflectivity Data using the PathFinder Detector Abridged SOP for Manually Aligning a Sample and Collecting Data using XRD Commander Scott A Speakman, Ph.D. MIT Center for
Bruker D8 HRXRD Collecting X-Ray Reflectivity Data using the PathFinder Detector Abridged SOP for Manually Aligning a Sample and Collecting Data using XRD Commander Scott A Speakman, Ph.D. MIT Center for
metal-organic compounds
 metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Monoclinic, P2 1 =n a = 11.6887 (5) Å b = 16.8061 (9) Å c = 11.7888 (5) Å = 103.757 (4) V = 2249.38 (18)
metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Monoclinic, P2 1 =n a = 11.6887 (5) Å b = 16.8061 (9) Å c = 11.7888 (5) Å = 103.757 (4) V = 2249.38 (18)
Importing and processing gel images
 BioNumerics Tutorial: Importing and processing gel images 1 Aim Comprehensive tools for the processing of electrophoresis fingerprints, both from slab gels and capillary sequencers are incorporated into
BioNumerics Tutorial: Importing and processing gel images 1 Aim Comprehensive tools for the processing of electrophoresis fingerprints, both from slab gels and capillary sequencers are incorporated into
Experimental. Crystal data
 organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 N-(Diphenylcarbamoyl)-N,N 0,N 0,N 00,N 00 - pentamethylguanidinium tetraphenylborate Ioannis Tiritiris Fakultät
organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 N-(Diphenylcarbamoyl)-N,N 0,N 0,N 00,N 00 - pentamethylguanidinium tetraphenylborate Ioannis Tiritiris Fakultät
Experimental. Crystal data. C 15 H 21 FN 3 O 4 P M r = Monoclinic, P2 1 =n a = (6) Å b = (4) Å c = (9) Å = 106.
 organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 A second monoclinic polymorph of N-[bis(morpholin-4-yl)phosphinoyl]- 4-fluorobenzamide with the P2 1 /n space
organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 A second monoclinic polymorph of N-[bis(morpholin-4-yl)phosphinoyl]- 4-fluorobenzamide with the P2 1 /n space
Operating Procedures for MICROCT1 Nikon XTH 225 ST
 Operating Procedures for MICROCT1 Nikon XTH 225 ST Ensuring System is Ready (go through to ensure all windows and tasks below have been completed either by you or someone else prior to mounting and scanning
Operating Procedures for MICROCT1 Nikon XTH 225 ST Ensuring System is Ready (go through to ensure all windows and tasks below have been completed either by you or someone else prior to mounting and scanning
User Operation of JEOL 1200 EX II
 **Log onto Computer** Open item program Start Up Procedure User Operation of JEOL 1200 EX II 1. If scope is not running, locate an electron microscopy technician (EMT) to find out why not. 2. Turn up brightness
**Log onto Computer** Open item program Start Up Procedure User Operation of JEOL 1200 EX II 1. If scope is not running, locate an electron microscopy technician (EMT) to find out why not. 2. Turn up brightness
Basic P-XRD instructions for Operating the Instrument
 Basic P-XRD instructions for Operating the Instrument Instrument Parts Incident Beam Optics (left arm) 1) X-ray source (Cu) i. Rest settings: 45 kv, 20mA ii. Run settings: 45 kv, 40mA 2) Monochromator
Basic P-XRD instructions for Operating the Instrument Instrument Parts Incident Beam Optics (left arm) 1) X-ray source (Cu) i. Rest settings: 45 kv, 20mA ii. Run settings: 45 kv, 40mA 2) Monochromator
Horiba Jobin-Yvon LabRam Raman Confocal Microscope (GERB 120)
 Horiba Jobin-Yvon LabRam Raman Confocal Microscope (GERB 120) Please contact Dr. Amanda Henkes for training requests and assistance: 979-862-5959, amandahenkes@tamu.edu Hardware LN 2 FTIR FTIR camera 1
Horiba Jobin-Yvon LabRam Raman Confocal Microscope (GERB 120) Please contact Dr. Amanda Henkes for training requests and assistance: 979-862-5959, amandahenkes@tamu.edu Hardware LN 2 FTIR FTIR camera 1
1.2. Make sure the viewing screen is covered (exposure to liquid N 2 may cause it to crack).
 FEI Tecnai F20 S/TEM: imaging in TEM mode Nicholas G. Rudawski ngr@ufl.edu (805) 252-4916 (352) 392-3077 Last updated: 01/21/18 1. Filling the cold trap (if needed) 1.1. Prior to use, the cold trap needs
FEI Tecnai F20 S/TEM: imaging in TEM mode Nicholas G. Rudawski ngr@ufl.edu (805) 252-4916 (352) 392-3077 Last updated: 01/21/18 1. Filling the cold trap (if needed) 1.1. Prior to use, the cold trap needs
Atomic and nuclear physics
 Atomic and nuclear physics X-ray physics Physics of the atomic shell LEYBOLD Physics Leaflets Investigating the energy spectrum of an x-ray tube as a function of the high voltage and the emission current
Atomic and nuclear physics X-ray physics Physics of the atomic shell LEYBOLD Physics Leaflets Investigating the energy spectrum of an x-ray tube as a function of the high voltage and the emission current
metal-organic compounds
 metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 a = 8.8035 (11) Å b = 18.138 (2) Å c = 20.966 (3) Å = 95.512 (2) V = 3332.4 (7) Å 3 Z =4 Mo K radiation
metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 a = 8.8035 (11) Å b = 18.138 (2) Å c = 20.966 (3) Å = 95.512 (2) V = 3332.4 (7) Å 3 Z =4 Mo K radiation
Olympus Digital Microscope Camera (DP70) checklist
 Smith College - July 2005 Olympus Digital Microscope Camera (DP70) checklist CONTENT, page no. Camera Information, 1 Startup, 1 Retrieve an Image, 2 Microscope Setup, 2 Capture, 3 Preview. 3 Color Balans,
Smith College - July 2005 Olympus Digital Microscope Camera (DP70) checklist CONTENT, page no. Camera Information, 1 Startup, 1 Retrieve an Image, 2 Microscope Setup, 2 Capture, 3 Preview. 3 Color Balans,
Optika ISview. Image acquisition and processing software. Instruction Manual
 Optika ISview Image acquisition and processing software Instruction Manual Key to the Instruction Manual IS is shortened name used for OptikaISview Square brackets are used to indicate items such as menu
Optika ISview Image acquisition and processing software Instruction Manual Key to the Instruction Manual IS is shortened name used for OptikaISview Square brackets are used to indicate items such as menu
Nature Methods: doi: /nmeth Supplementary Figure 1. Resolution of lysozyme microcrystals collected by continuous rotation.
 Supplementary Figure 1 Resolution of lysozyme microcrystals collected by continuous rotation. Lysozyme microcrystals were visualized by cryo-em prior to data collection and a representative crystal is
Supplementary Figure 1 Resolution of lysozyme microcrystals collected by continuous rotation. Lysozyme microcrystals were visualized by cryo-em prior to data collection and a representative crystal is
Alibre Design Tutorial: Loft, Extrude, & Revolve Cut Loft-Tube-1
 Alibre Design Tutorial: Loft, Extrude, & Revolve Cut Loft-Tube-1 Part Tutorial Exercise 5: Loft-Tube-1 [Complete] In this Exercise, We will set System Parameters first, then part options. Then, in sketch
Alibre Design Tutorial: Loft, Extrude, & Revolve Cut Loft-Tube-1 Part Tutorial Exercise 5: Loft-Tube-1 [Complete] In this Exercise, We will set System Parameters first, then part options. Then, in sketch
metal-organic compounds
 metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Dichlorido(3-phenylindenylidene)bis- (triphenylphosphane)ruthenium(ii) tetrahydrofuran disolvate Jan W.
metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Dichlorido(3-phenylindenylidene)bis- (triphenylphosphane)ruthenium(ii) tetrahydrofuran disolvate Jan W.
An Activity in Computed Tomography
 Pre-lab Discussion An Activity in Computed Tomography X-rays X-rays are high energy electromagnetic radiation with wavelengths smaller than those in the visible spectrum (0.01-10nm and 4000-800nm respectively).
Pre-lab Discussion An Activity in Computed Tomography X-rays X-rays are high energy electromagnetic radiation with wavelengths smaller than those in the visible spectrum (0.01-10nm and 4000-800nm respectively).
For customers in Canada This Class B digital apparatus meets all requirements of the Canadian Interference-Causing Equipment Regulations.
 User manual For customers in North and South America For customers in USA This device complies with Part 15 of the FCC rules. Operation is subject to the following two conditions: (1) This device may not
User manual For customers in North and South America For customers in USA This device complies with Part 15 of the FCC rules. Operation is subject to the following two conditions: (1) This device may not
1. Creating geometry based on sketches 2. Using sketch lines as reference 3. Using sketches to drive changes in geometry
 4.1: Modeling 3D Modeling is a key process of getting your ideas from a concept to a read- for- manufacture state, making it core foundation of the product development process. In Fusion 360, there are
4.1: Modeling 3D Modeling is a key process of getting your ideas from a concept to a read- for- manufacture state, making it core foundation of the product development process. In Fusion 360, there are
metal-organic compounds
 metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Hexaaquacadmium(II) bis{[n-(2- oxidobenzylidene)glycyl-l-leucinato]- cuprate(ii)} dihydrate Guolin Zhang,
metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Hexaaquacadmium(II) bis{[n-(2- oxidobenzylidene)glycyl-l-leucinato]- cuprate(ii)} dihydrate Guolin Zhang,
SEM Training Notebook
 SEM Training Notebook Lab Manager: Dr. Perry Cheung MSE Fee-For-Service Facility Materials Science and Engineering University of California, Riverside December 21, 2017 (rev. 3.4) 1 Before you begin Complete
SEM Training Notebook Lab Manager: Dr. Perry Cheung MSE Fee-For-Service Facility Materials Science and Engineering University of California, Riverside December 21, 2017 (rev. 3.4) 1 Before you begin Complete
ThermaViz. Operating Manual. The Innovative Two-Wavelength Imaging Pyrometer
 ThermaViz The Innovative Two-Wavelength Imaging Pyrometer Operating Manual The integration of advanced optical diagnostics and intelligent materials processing for temperature measurement and process control.
ThermaViz The Innovative Two-Wavelength Imaging Pyrometer Operating Manual The integration of advanced optical diagnostics and intelligent materials processing for temperature measurement and process control.
Olympus xcellence Software - basic user guide
 Olympus xcellence Software - basic user guide This is a basic overview of setting up time lapse experiments using Olympus's xcellence software on BIU's IX81 inverted phase contrast system - the software
Olympus xcellence Software - basic user guide This is a basic overview of setting up time lapse experiments using Olympus's xcellence software on BIU's IX81 inverted phase contrast system - the software
LECTURE 10. Dr. Teresa D. Golden University of North Texas Department of Chemistry
 LECTURE 10 Dr. Teresa D. Golden University of North Texas Department of Chemistry Components for the source include: -Line voltage supply -high-voltage generator -x-ray tube X-ray source requires -high
LECTURE 10 Dr. Teresa D. Golden University of North Texas Department of Chemistry Components for the source include: -Line voltage supply -high-voltage generator -x-ray tube X-ray source requires -high
Experimental. Crystal data. C 30 H 32 N 2 O 7 CH 4 O M r = Monoclinic, P2 1 a = (4) Å b = (3) Å c = (5) Å = 105.
 organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 9-{[4-(Dimethylamino)benzyl]amino}- 5-(4-hydroxy-3,5-dimethoxyphenyl)- 5,5a,8a,9-tetrahydrofuro[3 0,4 0 :6,7]-
organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 9-{[4-(Dimethylamino)benzyl]amino}- 5-(4-hydroxy-3,5-dimethoxyphenyl)- 5,5a,8a,9-tetrahydrofuro[3 0,4 0 :6,7]-
LumaSpec 800S User Manual
 LumaSpec 800S User Manual Worldwide distribution VERSION 09112014 Prior Scientific, Ltd Cambridge, UK Prior Scientific, Inc Rockland, MA. USA Prior Scientific, GmbH Jena, Germany Prior Scientific KK Tokyo,
LumaSpec 800S User Manual Worldwide distribution VERSION 09112014 Prior Scientific, Ltd Cambridge, UK Prior Scientific, Inc Rockland, MA. USA Prior Scientific, GmbH Jena, Germany Prior Scientific KK Tokyo,
Beginner s Guide to SolidWorks Alejandro Reyes, MSME Certified SolidWorks Professional and Instructor SDC PUBLICATIONS
 Beginner s Guide to SolidWorks 2008 Alejandro Reyes, MSME Certified SolidWorks Professional and Instructor SDC PUBLICATIONS Schroff Development Corporation www.schroff.com www.schroff-europe.com Part Modeling
Beginner s Guide to SolidWorks 2008 Alejandro Reyes, MSME Certified SolidWorks Professional and Instructor SDC PUBLICATIONS Schroff Development Corporation www.schroff.com www.schroff-europe.com Part Modeling
CHROMACAL User Guide (v 1.1) User Guide
 CHROMACAL User Guide (v 1.1) User Guide User Guide Notice Hello and welcome to the User Guide for the Datacolor CHROMACAL Color Calibration System for Optical Microscopy, a cross-platform solution that
CHROMACAL User Guide (v 1.1) User Guide User Guide Notice Hello and welcome to the User Guide for the Datacolor CHROMACAL Color Calibration System for Optical Microscopy, a cross-platform solution that
USING LEICA AS LASER MICRODISSECTION (LMD6000) MICROSCOPE Written By Jungim Hur
 USING LEICA AS LASER MICRODISSECTION (LMD6000) MICROSCOPE Written By Jungim Hur Digital Video Camera Eyepieces Laser module Laser safety UV shield Specimen holder Smart move control LEICA CTR6500 electronics
USING LEICA AS LASER MICRODISSECTION (LMD6000) MICROSCOPE Written By Jungim Hur Digital Video Camera Eyepieces Laser module Laser safety UV shield Specimen holder Smart move control LEICA CTR6500 electronics
SEM Training Notebook
 SEM Training Notebook Lab Manager: Dr. Perry Cheung MSE Fee-For-Service Facility Materials Science and Engineering University of California, Riverside March 8, 2018 (rev. 3.5) 1 Before you begin Complete
SEM Training Notebook Lab Manager: Dr. Perry Cheung MSE Fee-For-Service Facility Materials Science and Engineering University of California, Riverside March 8, 2018 (rev. 3.5) 1 Before you begin Complete
X-ray investigation of crystal structures / Laue method with digital X-ray detector (XRIS) (Item No.: P )
 X-ray investigation of crystal structures / Laue method with digital X-ray detector (XRIS) (Item No.: P2541602) Curricular Relevance Area of Expertise: Physik Education Level: Hochschule Topic: Moderne
X-ray investigation of crystal structures / Laue method with digital X-ray detector (XRIS) (Item No.: P2541602) Curricular Relevance Area of Expertise: Physik Education Level: Hochschule Topic: Moderne
Instruction Manual for HyperScan Spectrometer
 August 2006 Version 1.1 Table of Contents Section Page 1 Hardware... 1 2 Mounting Procedure... 2 3 CCD Alignment... 6 4 Software... 7 5 Wiring Diagram... 19 1 HARDWARE While it is not necessary to have
August 2006 Version 1.1 Table of Contents Section Page 1 Hardware... 1 2 Mounting Procedure... 2 3 CCD Alignment... 6 4 Software... 7 5 Wiring Diagram... 19 1 HARDWARE While it is not necessary to have
Notes on the Configuration, Installation, and Use of the 4Spots ImageJ plug-in
 Configuring 4spots The 4spots plugin will quickly measure the length of two g-vectors, as well as the angle between them. It will further use these values to compute associated d-spacings. This is accomplished
Configuring 4spots The 4spots plugin will quickly measure the length of two g-vectors, as well as the angle between them. It will further use these values to compute associated d-spacings. This is accomplished
ECEN. Spectroscopy. Lab 8. copy. constituents HOMEWORK PR. Figure. 1. Layout of. of the
 ECEN 4606 Lab 8 Spectroscopy SUMMARY: ROBLEM 1: Pedrotti 3 12-10. In this lab, you will design, build and test an optical spectrum analyzer and use it for both absorption and emission spectroscopy. The
ECEN 4606 Lab 8 Spectroscopy SUMMARY: ROBLEM 1: Pedrotti 3 12-10. In this lab, you will design, build and test an optical spectrum analyzer and use it for both absorption and emission spectroscopy. The
1 Sketching. Introduction
 1 Sketching Introduction Sketching is arguably one of the more difficult techniques to master in NX, but it is well-worth the effort. A single sketch can capture a tremendous amount of design intent, and
1 Sketching Introduction Sketching is arguably one of the more difficult techniques to master in NX, but it is well-worth the effort. A single sketch can capture a tremendous amount of design intent, and
Welcome 1. Precaution
 Table of Contents EN Precaution....2 Preparation.. 4 Standard accessories....4 Parts Names & Functions...5 Computer System requirements.... 6 Technical Specifications 7 Install the software.. 7 Start Microscope.8
Table of Contents EN Precaution....2 Preparation.. 4 Standard accessories....4 Parts Names & Functions...5 Computer System requirements.... 6 Technical Specifications 7 Install the software.. 7 Start Microscope.8
Structural Biology. Single crystal X-ray diffraction and SAXS. Systems designed for structural biology
 Structural Biology Single crystal X-ray diffraction and SAXS Systems designed for structural biology Rigaku Oxford Diffraction THE POWER OF SYNERGY Combining the single crystal groups from Rigaku and Oxford
Structural Biology Single crystal X-ray diffraction and SAXS Systems designed for structural biology Rigaku Oxford Diffraction THE POWER OF SYNERGY Combining the single crystal groups from Rigaku and Oxford
FEI Falcon Direct Electron Detector. Best Practice Document
 FEI Falcon Direct Electron Detector Best Practice Document 2 1. Introduction FEI Falcon Direct Electron Detector Best Practice Application Guide The FEI Falcon Detector is based on direct electron detection
FEI Falcon Direct Electron Detector Best Practice Document 2 1. Introduction FEI Falcon Direct Electron Detector Best Practice Application Guide The FEI Falcon Detector is based on direct electron detection
ISCapture User Guide. advanced CCD imaging. Opticstar
 advanced CCD imaging Opticstar I We always check the accuracy of the information in our promotional material. However, due to the continuous process of product development and improvement it is possible
advanced CCD imaging Opticstar I We always check the accuracy of the information in our promotional material. However, due to the continuous process of product development and improvement it is possible
K-band Waveguide BPF Design using Agilent EMPro Anurag Bhargava Application Consultant Agilent EEsof EDA
 K-band Waveguide BPF Design using Agilent EMPro 2013 Anurag Bhargava Application Consultant Agilent EEsof EDA Filter Specifications Center Frequency (Fc): 25 GHz 3dB Bandwidth: 150 MHz Rejection: 40 db
K-band Waveguide BPF Design using Agilent EMPro 2013 Anurag Bhargava Application Consultant Agilent EEsof EDA Filter Specifications Center Frequency (Fc): 25 GHz 3dB Bandwidth: 150 MHz Rejection: 40 db
ejs002_cm2_co ejs002_stak1_co ejs002_ofit_co ejs002_stak2_co
 ejs002_cm2_co ejs002_cm3_co ejs002_cm1_co ejs002_stak1_co ejs002_ofit_co ejs002_stak2_co ejs002_t1a_co ejs002_t1b_co ejs002_t2a_co ejs002_t2b_co ejs002_v12 1 9 Feb 2004 Acta Cryst. (2003). C59,
ejs002_cm2_co ejs002_cm3_co ejs002_cm1_co ejs002_stak1_co ejs002_ofit_co ejs002_stak2_co ejs002_t1a_co ejs002_t1b_co ejs002_t2a_co ejs002_t2b_co ejs002_v12 1 9 Feb 2004 Acta Cryst. (2003). C59,
AQA P3 Topic 1. Medical applications of Physics
 AQA P3 Topic 1 Medical applications of Physics X rays X-ray properties X-rays are part of the electromagnetic spectrum. X-rays have a wavelength of the same order of magnitude as the diameter of an atom.
AQA P3 Topic 1 Medical applications of Physics X rays X-ray properties X-rays are part of the electromagnetic spectrum. X-rays have a wavelength of the same order of magnitude as the diameter of an atom.
