QMedical ANS & Vascular HW10 Technical Specifications
|
|
|
- Brett Nicholson
- 5 years ago
- Views:
Transcription
1 QMedical ANS & Vascular HW10 Technical Specifications 1
2 GENERAL TECHNICAL DATA Note: Unless otherwise indicated, all specifications are subject to change without notice. Temperature range Operation Storage Transport USB communication. Power Supply Voltage Setup location, maximum height above mean sea level Atmospheric Pressure Device Box ingress protection Air humidity Operation Storage Transport Medical device in accordance with Directive 93/42/EEC EN : +10 C to +40 C (50 to 104 F) 40 C to +70 C ( 40 to 158 F) 40 C to +70 C ( 40 to 158 F) 5V DC from the Computer/Notebook USB port. QMedical USB communication power consumption not more than: 5V DC/200mA DC. The Computer/Notebook should be IEC certified (or equivalent) The power supply is delivered via XP Power VEP Series Power Supply VEP15US05 (XP Power, 990 Benecia Avenue, Sunnyvale, CA 94085, USA) Type: Medical Grade Supply Rated Mains Supply Voltage: V~, 50/60Hz, 0.5A Output: +5.0V, 2.0A Protection Class 2 Continuous duty 3000m 50kPa to 106kPa IPX1 15% 85% non condensing 0% 95% non condensing 0% 95% non condensing Class lla Insulated device, protection class II Electric medical device, Defibrillator proof Type CF Degree of protection against harmful ingress of water Degree of safety of application in the presence of a flammable anesthetic mixture with air or with oxygen or nitrous oxide Method(s) of sterilization or disinfection recommended by the manufacturer Biocompatibility Ordinary Equipment(enclosed equipment without protection against ingress of water) Equipment not suitable for use in the presence of a flammable anesthetic mixture with air or with oxygen or nitrous oxide Not applicable The parts of the product described in this operator manual, including all accessories, that come in contact with the patient during the intended use, fulfill the biocompatibility requirements of the applicable standards ISO If you have questions in this matter, please contact Medeia. Information Technologies or its representatives. Replaceable fuse F 1.0AL 250V, (FSF 5x20, ) Device LED Indicator Green Device is powered on and ready for use, Doppler probe is ON. Blinking Blue Device is measuring, LED Blinking with the patient Heart Rate 2
3 ECG TECHNICAL DATA ECG AMPLIFIER Simultaneous, Synchronous Registration Of All 9 Active Electrode Signals (= 12 Standard Leads) SAMPLING FREQUENCY 100/200/400 Hz; default 200Hz DIGITAL RESOLUTION 5 µv DYNAMIC RANGE MAX. ELECTRODE POTENTIAL TIME CONSTANT FREQUENCY RESPONSE ± 10 mvac ± 300 mvdc > 3.2 s ELECTRODE OFFSET ± 300mV TOLERANCE NONLINEARITY < 2% INPUT IMPEDANCE INPUT BIAS CURRENT LINE FREQUENCY FILTER LOW PASS HIGH PASS PATIENT LEAKAGE CURRENT PATIENT INPUT COMMON MODE REJECTION LEADS OFF INDICATORS GAIN/SENSITIVITY SWEEP SPEED INPUT RANGE A/D CONVERSION EMG FILTER T WAVE AMPLITUDE (AT) HEART RATE RANGE HEART RATE UPDATE HEART RATE AVERAGING AND UPDATING HEART RATE RESOLUTION HEART RATE ACCURACY HEART RATE AND QRS DETECTION STABILIZATION PERIOD RESPONSE TIME OF HEART RATE METER TO CHANGE IN HEART RATE ISOLATION TIME CONSTANT PACEMAKER DETECTION 0.05 to 150 Hz ( 3 db) > 10 MOhms ~ 0,1μА Distortion free suppression of superimposed 50 or 60 Hz sinusoidal interferences; default AutoDetect 50, 100 or 150Hz; default 100Hz 0.05Hz or 3.4Hz; default 3.4Hz < 10 µa Fully floating and isolated, defibrillation protected. 80dB (minimum) 100dB (with filter) Connection status for each lead is shown on "Recording ECG" screen 10 mm/mv 25 mm/s ± 5 mv 16bit 35Hz ( 3dB) / 25Hz ( 3dB) T wave amplitude >1.2 times of R wave amplitude can be rejected bpm every 1.5 seconds 2 s + the delay for the occurs of the next QRS pulse 1bpm ± 1bpm < 20s HR change from 80 to 120 bpm: 9 to 12 seconds HR change from 80 to 40 bpm: 9 to 13 seconds Isolated from ground related circuits by >4 kv rms, 5.5 kv peak The pacemaker pulse rejection capability includes pulses between +/ 2mV and +/ 700mV amplitude, with widths between 1ms and 2ms and overshoot from 0 to 100ms. The Device is not capable of rejecting A V Sequential pacemaker pulses. 3
4 PHOTOPLETHYSMOGRAPHY (PPG) Wavelength: 940 nm Artifact Rejection: Synchronous demodulation, AC coupled output Operating Frequency: 100Hz, 200Hz, 400Hz Operating Resolution: 12bit Cable length: 6.5 ft Pulse Rate Range from PPG (during no motion conditions): 30 to 220 bpm ±3 digits Pulse Rate Range from PPG (during Motion conditions): 30 to 220 bpm ±3 digits PPG waveform: non normalized. PPG waveform is present on display for visual signal quality check if require. PPG Signal quality inadequacy: It detect if a patient finger/toe is attach to the sensor. If not a signal is bad warning appear on display. OXYGEN SATURATION Oxygen Saturation Rang (%SpO2): 70 to 100% Maximum Measurement Wavelengths: Red: mw; Infrared: mw SpO2 Accuracy: 70 to 80% +/ 3 digits SaO2; 80 to 100% +/ 2 digits SaO2; SpO2 Accuracy for Neonate: 70 to 100% +/ 3 digits SaO2; SpO2 Update cycle: every 6s SpO2 detection stabilization period: 15s SpO2 Measuring mode: two wavelength lite absorption method Maximum application time per patient and site: no more than 30 min NOTE: HW10 device and sensor has been validated for no motion accuracy in human blood studies on healthy adult male and female volunteers with light to dark skin pigmentation in induced hypoxia studies in the range of % SpO2 against a laboratory Oximeter and ECG monitor. 1% was added to account for the properties of fetal hemoglobin. This variation equals plus or minus one standard deviation which encompasses 68% of the population. WARNING! The HW10 SpO2 can be used during defibrillation, but the readings may be inaccurate for up to 20 seconds. WARNING! Patient Safety If a sensor is damaged in any way, discontinue use immediately. WARNING! Do not place containers containing liquids on or near the HW10 device. Liquids spilled on the HW10 may cause it to perform inaccurately or fail. WARNING! Do not expose the HW10 to excessive moisture such as direct exposure to rain. Excessive moisture can cause the HW10 to perform inaccurately or fail. WARNING! Do not place the HW10 where the controls can be changed by the patient. WARNING! A functional tester cannot be utilized to assess the accuracy of the HW10 SpO2 or any sensors. WARNING! Severe anemia may cause erroneous SpO2 readings. WARNING! Do not place the HW10 or accessories in any position that might cause it to fall on the patient. Do not lift the HW10 by the patient cable. WARNING! Interfering Substances: SpO2 is a functional calculation of arterial oxygen saturation. Carboxyhemoglobin and methemoglobin may erroneously increase SpO2 readings. The level of increase is approximately equal to the amount of carboxyhemoglobin and/or methemoglobin present. Dyes, or any substance containing dyes, that change usual blood pigmentation may cause erroneous readings. WARNING! Do not connect/use other than provided SpO2/PPG sensor with that equipment, readings may be inaccurate. SpO2/PPG probes are designed for use with that specific equipment only; NOTE: The information about wavelength range can be especially useful to some clinicians. NOTE: Because PULSE OXIMETER EQUIPMENT measurements are statistically distributed, only about two thirds of PULSE OXIMETER EQUIPMENT measurements can be expected to fall within ±Arms of the value measured by a CO OXIMETER. When a PULSE OXIMETER MONITOR is suitable for use with a variety of PULSE OXIMETER PROBES, SpO2 ACCURACY information shall be made available for each type of PULSE OXIMETER PROBE. NOTE: Internal company technical personal need to refer to Performance Tests Validation and SpO2 Accuracy Validation Report for product final validation and verification before supply to the client. SpO2 measurement accuracy is based on clinical studies using Secondary standard sensors on volunteer subjects. Samples measurements were analyzed simultaneously. 4
5 BLOOD PRESSURE (NIBP) Number of Blood Pressure: 2, 3 or 4 Cuff pressure Range: mmhg Measuring range: mmhg Accuracy: +/ 2 mmhg mmhg, +/ 2% above 200mmHg Safety: Pressure released if greater than 255 mmhg Pressure released if held above 100 mmhg beyond 3 minutes Pressure released if held between mmhg beyond 5 minutes* (*Operator may over ride for exam purposes) Redundant Control Bleed Rate: 2.5 mmhg/sec nominal PVR: 0.16 to 12.5 Hz bandwidth, AC Coupled Cuff size: S, M, L, XL CAUTION! During unlikely event of over pressure of the cuffs or retaining pressure long time use the Red Button on the front panel of the device to deflate the blood pressure cuff or disconnect the cuffs from the device. CAUTION! BLOOD PRESSURE reading can be affected by the measurement site, the position of the PATIENT (standing, sitting, lying down), exercise, or the PATIENT'S physiologic condition. Environmental or operational factors which can affect the performance of the Blood Pressure reading (e.g. common arrhythmias such as atrial or ventricular premature beats or atrial fibrillation, arterial sclerosis, poor perfusion, diabetes, age, pregnancy, pre eclampsia, renal diseases, Patient motion, trembling, shivering); ULTRASOUND DOPPLER PROBE Doppler Type: Bi Directional, Continuous Wave (CW) Audio Bandwidth: >4 KHz Waveform Display: Digital Mean Frequency Estimator Separate Forward and Reverse Flow Waveforms Available Probe Frequencies: 8MHz, 4MHz Displayed Waveform Amplitude Accuracy: +/ 10% Speaker Output: > 0.25 W Speaker Type: 2 inch, 5 W Cable length: 6.5 ft Volume Control: Continuously variable with mute Waterproof Obstetrical Probe FDA 510k Clearance: K Low Output Summary Table (for systems with no transducers having global maximum index values exceeding 1.0) Transducer Model (MHz) I spta.3 (mw/cm2) TI Type TI Value MI I sppa.3 (W/cm2) TIS 0.01 CW 4MHz TIB TIS CW 4MHz TIB TIS 0.08 CW 8MHz Overall Sensitivity 4MHz Probe 5
6 CONTROLS AND INDICATORS 1) 12 Lead or 3 lead ECG Electrode Cable 2) Reusable Finger/Toe Photoplethysmography (PPG/SpO2) Sensor 1 3) Reusable Finger/Toe Photoplethysmography (PPG/SpO2) Sensor 2 4) Doppler Bi Directional, Continuous Wave (CW) Probe (4MHz or 8MHz) 5) Non Invasive Blood Pressure Cuff 1 6) Non Invasive Blood Pressure Cuff 2 7) Event Button or Blood Pressure Relief button 8) LED Indicator: Green Device is powered on and ready for use; Blinking Blue Device is measuring (Doppler probe is ON), LED is blinking with the patient Heart Rate. 9) Power Supply: +5V, 1.6A 10) Device On/Off Button 11) Reusable USB Communication cable: USB 2.0 Cable Male A/B 6Ft 6
7 EMS SAFETY EMC Notice QMEDICAL generates, uses and can radiate radio frequency energy. If not set up correctly and used in accordance with the instructions in this manual, electromagnetic interference may result. The equipment has been tested and complies with the limits set forth in IEC for Medical Products. These limits provide reasonable protection against electromagnetic interference when operated in the intended environments (e.g. hospitals, research laboratories, etc.) described in this manual. MRI Notice This device contains components whose operation can be affected by intense electromagnetic fields. Do not operate the device in an MRI environment or in the vicinity of high frequency surgical diathermy equipment, defibrillators or short wave therapy equipment. Electromagnetic interference could disrupt the operation of this device. Intended Use Notice QMedical device is intended for detection of blood flow in veins and arteries and as an aid for the diagnosis of peripheral vascular disease. The system is intended for use on patients in medical clinics, healthcare practices and in out patient departments of hospitals. Manufacturer s declaration electromagnetic emissions The QMEDICAL System is intended for use in the electromagnetic environment specified below. The customer or user of the QMEDICAL System should assure that it is used in such an environment. Emissions test Compliance Electromagnetic environment guidance Radio Frequency emissions Group 1 The QMEDICAL uses Radio Frequency CISPR 11 energy only for its internal function. Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment. Radio Frequency emissions CISPR 11 Harmonic emissions IEC Voltage fluctuations/flicker emissions IEC Class CF Not applicable Not applicable The QMEDICAL is suitable for use in all establishments, including domestic establishments and those directly connected to the public low voltage power supply network that supplies buildings used for domestic purposes. Manufacturer s declaration electromagnetic immunity The QMEDICAL is intended for use in the electromagnetic environment specified below. The customer or the user of the QMEDICAL should assure that it is used in such an environment. Immunity test IEC test level Compli ance level Electrostatic discharge (ESD) IEC Radiated, radio frequency, electromagnetic field immunity test IEC ±2,4,6 kv contact direct ±2,4,6 kv contact indirect ±2,4,8 kv air direct 1/Frequency Mhz strength 3V/m Modulation 80% AM (2Hz) dwell time 2.8 sec. 2/ Frequency MHz strength 3V/m Modulation 80% AM (2Hz) dwell time 2.8 sec. 3/ Frequency MHz strength 3V/m Modulation 80% AM (2Hz ) dwell time 2.8 sec. B A Electromagnetic environment guidance Floors should be wood, concrete or ceramic tile. If floors are covered with synthetic material the relative humidity should be at least 15%. Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment. Immunity to conducted disturbances, induced by radiofrequency fields IEC Power frequency (50/60 Hz) magnetic field IEC Frequency MHz Vrms 3 Modulation 80% AM (2Hz) Dwell time 2.8 sec. Frequency 50 and 60 Hz, 3 A/m A A Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment. Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment. 7
8 STANDARD CERTIFICATION Classification: Class II Equipment Powered Equipment External power supply Degree of protection against ingress of water: Ordinary (not rated) Degree of Safety in Presence of Flammable Gases: Equipment not suitable for use in presence of flammable gases Designed and tested to meet: IEC , IEC :2012, AAMI :2011, IEC :2007, AAMI : A1:2013, ISO :2011. EMC Classification: Class A This equipment was tested to applicable standards for electromagnetic interference. If interference occurs, attempt to eliminate the source of the interference or increase the distance between the QMedical and the source of the interference. WARNING: Connect the QMedical only to equipment that meets the appropriate standards. The QMedical and its External Power Supply are a medical system. The External Power Supply must meet IEC construction requirements for Reinforced Attention: Consult Accompanying Documents, Additional technical information is available upon customer request. FDA 510k Clearance: K080884, K083735, K EU CE mark: 0120 (SGS), Directive 93/42/EEC, ISO 13485:2003 REPORT PARAMETERS: Artery Assessment: Endothelial Dysfunction, Pulse Wave Velocity, Peripheral Augmentation Index, Central Aortic Systolic Pressure, Segmental Ankle Brachial Indices (ABI) and Toe Brachial Index (TBI), PPG, PVR, PTT. Cardiac Autonomic Function Testing (ANS): Heart rate variability Analysis and Cardiac Autonomic Reflex Tests (Autonomic Tonus, Autonomic Balance, Valsalva Ratio, Deep Breathing Reflex, E/I Ratio, K30/15 Ratio and Beat to Beat Systolic blood pressure response difference while standing or tilt). ECG Assessment: 3 or 12 Leads ECG Assessment, Ventricular Extrasystoles (PVC), Atrial Extrasystoles (PAC), QT Variability Overall Health Level Assessment: Accumulative Mental Stress Index, Accumulative Physical Stress Index, Overall Health Level Sudomotor Assessment: (QBioScan/SudoCheck) Sympathetic skin response(ssr), Quantitative Sudomotor Axon Reflex Test (QSART) 8
9 QBioscan SudoCheck HW4 Technical Specifications 9
10 SYMPATHETIC SKIN RESPONSE (SSR) SPECIFICATION Note: Unless otherwise indicated, all specifications are subject to change without notice. Classification Regulation Product Code Indication Operating Characteristics , Class II GZO QBioScan/SudoCheck is indicated for the measurement of galvanic/sympathetic skin response and QSART to aid in the assessment of the sudomotor function. The device is intended for use on the general adult population in medical clinics, healthcare practices and out patient departments of hospitals. Measures difference in skin conductance. Used to provide feedback to physicians, not to diagnose. Skin Contact Pads measure skin conductance. Electrode Surface Area Hand: 384 cm 2 Electrode Placement (Anatomic Sites) Electrode Materials Foot: 384 cm 2 Head: 7 cm 2 (optional) Hands, Feet, Forehead Head Electrode: Stainless Steel with Ag/AgCl layer (cleared electrode) Hand and Foot Electrode: AISI 304 Stainless Steel Skin Conductance µs/cm 2 Measurement Range Skin Conductance Resolution 1 ns/cm 2 Acquisition Duration (total) 120, 300 or 600 seconds Electrical Output to the skin 4 V max Electrical Output Frequency Continuous Electrical Output Unit 1 second Duration Power Density (at electrode) 0.01 µa/mm 2 User Display Computer Display User Control Computer provided with system Audible Indicators Internal Computer Speaker and Optional Headphone Output Report 4 average values of 12 STC measurements: Two STC for each Hand: Average value of a minimum of 6 STC measurements Two STC for each Foot: Average value of a minimum of 6 STC measurements Head: Average value of a minimum of 6 STC measurements Interface USB from laptop Power Source 5 V provided by USB Electrical Safety Standards IEC EN Electrical Safety Classification Class II Type BF Applied Part 10
11 GENERAL TECHNICAL DATA Temperature range Operation Storage Transport Power Supply Voltage Setup location, maximum height above mean sea level Atmospheric Pressure Air humidity Operation Storage Transport Medical device in accordance with Directive 93/42/EEC EN : +10 C to +40 C (50 to 104 F) 40 C to +70 C ( 40 to 158 F) 40 C to +70 C ( 40 to 158 F) 5V DC from the Computer/Notebook USB port. QBioscan device power consumption not more than: 5V DC/100mA DC. The Computer/Notebook should be IEC certified (or equivalent) 3000m 50kPa to 106kPa 30% 70% non condensing 0% 95% non condensing 0% 95% non condensing Class lla Insulated device, protection class II Electric medical device, type BF Measuring range Skin Resistance: 1KOhms 100KOhms Measurement Accuracy Skin Resistance: 5% Non replaceable fuse F1: F 500mAH, 32V (USF A5) STANDARD CERTIFICATION Classification: Class II Equipment Powered Equipment External power supply Degree of protection against ingress of water: Ordinary (not rated) Degree of Safety in Presence of Flammable Gases: Equipment not suitable for use in presence of flammable gases Designed and tested to meet: IEC , IEC :2012, EMC Classification: Class A This equipment was tested to applicable standards for electromagnetic interference. If interference occurs, attempt to eliminate the source of the interference or increase the distance between the QMedical and the source of the interference. WARNING: Connect the QMedical only to equipment that meets the appropriate standards. The QMedical and its External Power Supply are a medical system. The External Power Supply must meet IEC construction requirements for Reinforced Attention: Consult Accompanying Documents, Additional technical information is available upon customer request. FDA 510k Clearance: K EU CE mark: 0120 (SGS), Directive 93/42/EEC, ISO 13485:
12 EMS SAFETY EMC Notice QBIOSCAN generates, uses and can radiate radio frequency energy. If not set up correctly and used in accordance with the instructions in this manual, electromagnetic interference may result. The equipment has been tested and complies with the limits set forth in IEC for Medical Products. These limits provide reasonable protection against electromagnetic interference when operated in the intended environments (e.g. hospitals, research laboratories, etc.) described in this manual. MRI Notice This device contains components whose operation can be affected by intense electromagnetic fields. Do not operate the device in an MRI environment or in the vicinity of high frequency surgical diathermy equipment, defibrillators or short wave therapy equipment. Electromagnetic interference could disrupt the operation of this device. Intended Use Notice QBioScan device is a medical device for the measurement of galvanic skin response. The system is intended for use on patients in medical clinics, healthcare practices and in out patient departments of hospitals. It should only be operated by properly trained personnel. The results obtained from the QBioscan System are used for information only and are not to be used as any diagnostic process. Manufacturer s declaration electromagnetic emissions The QBIOSCAN System is intended for use in the electromagnetic environment specified below. The customer or user of the QBIOSCAN System should assure that it is used in such an environment. Emissions test Compliance Electromagnetic environment guidance Radio Frequency emissions Group 1 The QBIOSCAN uses Radio Frequency CISPR 11 energy only for its internal function. Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment. Radio Frequency emissions CISPR 11 Harmonic emissions IEC Voltage fluctuations/flicker emissions IEC Class B Not applicable Not applicable The QBIOSCAN is suitable for use in all establishments, including domestic establishments and those directly connected to the public low voltage power supply network that supplies buildings used for domestic purposes. Manufacturer s declaration electromagnetic immunity The QBIOSCAN is intended for use in the electromagnetic environment specified below. The customer or the user of the QBIOSCAN should assure that it is used in such an environment. Immunity test IEC test level Compli ance level Electrostatic discharge (ESD) IEC Radiated, radio frequency, electromagnetic field immunity test IEC Immunity to conducted disturbances, induced by radiofrequency fields IEC Power frequency (50/60 Hz) magnetic field IEC ±2,4,6 kv contact direct ±2,4,6 kv contact indirect ±2,4,8 kv air direct 1/Frequency Mhz strength 3V/m Modulation 80% AM (2Hz) dwell time 2.8 sec. 2/ Frequency MHz strength 3V/m Modulation 80% AM (2Hz) dwell time 2.8 sec. 3/ Frequency MHz strength 3V/m Modulation 80% AM (2Hz ) dwell time 2.8 sec. Frequency MHz Vrms 3 Modulation 80% AM (2Hz) Dwell time 2.8 sec. Frequency 50 and 60 Hz, 3 A/m B A A A Electromagnetic environment guidance Floors should be wood, concrete or ceramic tile. If floors are covered with synthetic material the relative humidity should be at least 30%. Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment. Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment. Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment. 12
13 Product Comparison QANS HW6C QMedical HW10 2B QDoppler HW10 4B D QBioscan HW4 QMedical Combo HW10 & HW4 SENSORS 3 Leads ECG Yes Yes Yes Yes 12 Leads ECG Yes Yes Yes PPG/SpO2 Yes (two) Yes (two) Yes (two) Yes (two) Blood Pressure Cuffs (NIBP) one (external) Yes (two) Yes (four) Yes (two or four) PVR Yes (two) Yes (four) Yes (two or four) Beat to Beat Systolic blood pressure calc Yes Yes Yes Respiration Flow Yes Yes Yes Doppler 4Mhz/8Mhz Yes (external) optional Galvanic/Sympathetic Skin Response Yes Yes Bioimpedance Yes Yes ARTERY ASSESSMENT: Endothelial Dysfunction Yes Yes Yes Yes Pulse Wave Velocity Yes Yes Yes Yes Peripheral Augmentation Index Yes Yes Yes Yes Central Aortic Systolic Pressure Yes Yes Yes Knee Brachial Indices Yes (two) optional Ankle Brachial Indices calc Yes (two) Yes (two) Yes (two) Toe Brachial Index Yes Yes Yes Yes PTT Yes Yes Yes Yes Exercise Vascular assessment Yes Yes Yes Doppler Assessment Yes optional CARDIAC AUTONOMIC FUNCTION TESTING (ANS): Heart rate variability Analysis Yes Yes Yes Yes Cardiac Autonomic Reflex Tests (Autonomic Tonus, Yes Yes Yes Yes Autonomic Balance, Valsalva Ratio, Deep Breathing, E/I Ratio, K30/15 Ratio). Beat to Beat Systolic blood pressure response calc Yes Yes Yes difference while standing or tilt Autonomic Baroreflex assessment Yes Yes Yes SUDOMOTOR ASSESSMENT: Sympathetic skin response(ssr) Yes Yes Quantitative Sudomotor Axon Reflex Test (QSART) Yes Yes Bio impedance Body Composition Yes Yes ECG ASSESSMENT: 3 Leads ECG Assessment Yes Yes Yes Yes 12 Leads ECG Assessment Yes Yes Yes Ventricular Extrasystoles (PVC) Yes Yes Yes Yes Atrial Extrasystoles (PAC) Yes Yes Yes Yes QT Variability Yes Yes Yes Yes OVERALL HEALTH LEVEL ASSESSMENT: Accumulative Mental Stress Index Yes Yes Yes Yes Accumulative Physical Stress Index Yes Yes Yes Yes Overall Health Level Yes Yes Yes Yes 13
NeuVision 500. Abundant and friendly display interface, multifold ECG display screen:
 NeuVision 500 Features This monitoring system may be used to monitor patient s 6 physiological parameters: ECG, respiratory rate, body temperature, non-invasive blood pressure (NIBP), pulse oxygen saturation
NeuVision 500 Features This monitoring system may be used to monitor patient s 6 physiological parameters: ECG, respiratory rate, body temperature, non-invasive blood pressure (NIBP), pulse oxygen saturation
D C 01/2019 3
 D-0117968-C 01/2019 3 4 D-0117968-C 01/2019 Screw Driver Screw Driver Unplug both the Red & Blue connectors. (see above) Place a small flat head screw driver on the small orange tabs and push down while
D-0117968-C 01/2019 3 4 D-0117968-C 01/2019 Screw Driver Screw Driver Unplug both the Red & Blue connectors. (see above) Place a small flat head screw driver on the small orange tabs and push down while
English
 English Specifications Type Power Source Vibration Frequency Maximum Output Power Consumption Water Pressure Lighting NE134 AC120V 50/60Hz AC230V 50/60Hz 28~32kHz 8W Max. 42VA 0.1~0.5MPa (1~5kgf/cm
English Specifications Type Power Source Vibration Frequency Maximum Output Power Consumption Water Pressure Lighting NE134 AC120V 50/60Hz AC230V 50/60Hz 28~32kHz 8W Max. 42VA 0.1~0.5MPa (1~5kgf/cm
BIODEX MULTI- JOINT SYSTEM
 BIODEX MULTI- JOINT SYSTEM CONFORMANCE TO STANDARDS 850-000, 840-000, 852-000 FN: 18-139 5/18 Contact information Manufactured by: Biodex Medical Systems, Inc. 20 Ramsey Road, Shirley, New York, 11967-4704
BIODEX MULTI- JOINT SYSTEM CONFORMANCE TO STANDARDS 850-000, 840-000, 852-000 FN: 18-139 5/18 Contact information Manufactured by: Biodex Medical Systems, Inc. 20 Ramsey Road, Shirley, New York, 11967-4704
Technical Data. Electrocardiograph ECG-1250K TD.ECG1250_L. This technical data may be revised or replaced by Nihon Kohden at any time without notice.
 Technical Data Electrocardiograph ECG-1250K This technical data may be revised or replaced by Nihon Kohden at any time without notice. TD.ECG1250_L Specifications ECG input Input impedance: 20 MΩ Electrode
Technical Data Electrocardiograph ECG-1250K This technical data may be revised or replaced by Nihon Kohden at any time without notice. TD.ECG1250_L Specifications ECG input Input impedance: 20 MΩ Electrode
Xpod Specification and Technical Information
 Xpod Specification and Technical Information NONIN Medical, Inc. 13700 1st Avenue North Plymouth, Minnesota 55441-5443 USA 763-553-9968 800-356-8874 (USA and Canada) Fax 763-553-7807 E-mail: info@nonin.com
Xpod Specification and Technical Information NONIN Medical, Inc. 13700 1st Avenue North Plymouth, Minnesota 55441-5443 USA 763-553-9968 800-356-8874 (USA and Canada) Fax 763-553-7807 E-mail: info@nonin.com
Central Blood Pressure Meter Model cbp301. Operating Manual
 Central Blood Pressure Meter Model cbp301 Operating Manual Document cbp301-009 Issue 4 September 2011 Contents Introduction... 3 Package Contents... 5 Warnings and Cautions... 6 Contraindications... 7
Central Blood Pressure Meter Model cbp301 Operating Manual Document cbp301-009 Issue 4 September 2011 Contents Introduction... 3 Package Contents... 5 Warnings and Cautions... 6 Contraindications... 7
OtoRead - Technical Specifications Page 0. Technical Specifications. OtoRead D A 2017/06
 OtoRead - Technical Specifications Page 0 Technical Specifications OtoRead D-0116698-A 2017/06 OtoRead - Technical Specifications Page 1 OtoRead TM Configuration Overview The OtoReadTM is available in
OtoRead - Technical Specifications Page 0 Technical Specifications OtoRead D-0116698-A 2017/06 OtoRead - Technical Specifications Page 1 OtoRead TM Configuration Overview The OtoReadTM is available in
3880 Technical Specifications_ DRAFT_A Page 1 of 11
 3880 Technical Specifications_ DRAFT_A Page 1 of 11 3880 3880 Technical Specifications_ DRAFT_A Page 2 of 11 Type: Color TFT resistive touchscreen Screen Size: 25.7 cm (10.1 inches) diagonal Pixels: 800
3880 Technical Specifications_ DRAFT_A Page 1 of 11 3880 3880 Technical Specifications_ DRAFT_A Page 2 of 11 Type: Color TFT resistive touchscreen Screen Size: 25.7 cm (10.1 inches) diagonal Pixels: 800
Biological Safety. Electromagnetic Compatibility (EMC) Observe the following precautions related to biological safety.
 Biological Safety Observe the following precautions related to biological safety. WARNING: Non-medical (commercial) grade peripheral monitors have not been verified or validated by SonoSite as being suitable
Biological Safety Observe the following precautions related to biological safety. WARNING: Non-medical (commercial) grade peripheral monitors have not been verified or validated by SonoSite as being suitable
INTRODUCTION. 4 SAFETY INSTRUCTIONS. 5 ABOUT THIS DEVICE. 6 FIRST OPERATION. 7 THE DISPLAY. 8 ATTACHING THE WRIST SLEEVE. 9 CORRECT MEASUREMENT.
 Table of contents INTRODUCTION.............................. 4 SAFETY INSTRUCTIONS........................ 5 ABOUT THIS DEVICE.......................... 6 FIRST OPERATION............................ 7
Table of contents INTRODUCTION.............................. 4 SAFETY INSTRUCTIONS........................ 5 ABOUT THIS DEVICE.......................... 6 FIRST OPERATION............................ 7
PHYSIOFLOW Q-LINK TM
 PHYSIOFLOW Q-LINK TM Service Manual Thursday, 20 October 2016 First placing on the market : 18 January 2012 User Manual PhysioFlow Q-Link 1/17 Table of contents 1. General Information... 3 About this manual...
PHYSIOFLOW Q-LINK TM Service Manual Thursday, 20 October 2016 First placing on the market : 18 January 2012 User Manual PhysioFlow Q-Link 1/17 Table of contents 1. General Information... 3 About this manual...
Technical Specifications Micromedical VisualEyes 505 by Interacoustics
 VisualEyes 505 - Technical Specifications Page 0 Technical Specifications Micromedical VisualEyes 505 by Interacoustics D-0115523-B 2018/02 VisualEyes 505 - Technical Specifications Page 1 Included and
VisualEyes 505 - Technical Specifications Page 0 Technical Specifications Micromedical VisualEyes 505 by Interacoustics D-0115523-B 2018/02 VisualEyes 505 - Technical Specifications Page 1 Included and
OEM III Module Specification and Technical Information
 OEM III Module Specification and Technical Information NONIN Medical, Inc. 13700 1st Avenue North Plymouth, Minnesota 55441-5443 USA 763-553-9968 800-356-8874 (USA and Canada) Fax 763-553-7807 E-mail:
OEM III Module Specification and Technical Information NONIN Medical, Inc. 13700 1st Avenue North Plymouth, Minnesota 55441-5443 USA 763-553-9968 800-356-8874 (USA and Canada) Fax 763-553-7807 E-mail:
: 0089 GTIN
 GTIN: 00894912002050 PRECAUTIONS PROBE Non-invasive probes are for transcutaneous use only Probe transducer tips are thin and delicate. Be careful not to drop or hit the probe tip. After use, protect the
GTIN: 00894912002050 PRECAUTIONS PROBE Non-invasive probes are for transcutaneous use only Probe transducer tips are thin and delicate. Be careful not to drop or hit the probe tip. After use, protect the
General Safety/EMC and Electrical Information for i-limb ultra and i-limb digits
 1. General Safety 1.1 The i-limb ultra and i-limb digits devices are electrical devices, which under certain circumstances could present an electrical shock hazard to the user. Please read the accompanying
1. General Safety 1.1 The i-limb ultra and i-limb digits devices are electrical devices, which under certain circumstances could present an electrical shock hazard to the user. Please read the accompanying
Powered Traction Unit OPERATION MANUAL
 Powered Traction Unit OPERATION MANUAL CONTENTS Symbols Safety precautions Symbol for CAUTION Symbol for CONSULT INSTRUCTIONS FOR USE Symbol for SERIAL NUMBER Symbol for CATALOGUE NUMBER Symbol for AUTHORISED
Powered Traction Unit OPERATION MANUAL CONTENTS Symbols Safety precautions Symbol for CAUTION Symbol for CONSULT INSTRUCTIONS FOR USE Symbol for SERIAL NUMBER Symbol for CATALOGUE NUMBER Symbol for AUTHORISED
The following symbol indicates that the device is MR-unsafe:
 The following symbol indicates that the device is MR-unsafe: MR Unsafe Do not use this equipment In the MRI scan room Patient must follow the doctor s instructions and should not perform a self-assessment
The following symbol indicates that the device is MR-unsafe: MR Unsafe Do not use this equipment In the MRI scan room Patient must follow the doctor s instructions and should not perform a self-assessment
D500. Defibrillator/Monitor NIBP. Temperature 1. Temperature 2 IBP 1 IBP 2. Capnography. Integrated Thermal Printer LCD
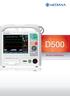 D500 Defibrillator/Monitor LCD Waveform & Text display NIBP Temperature 1 Temperature 2 IBP 1 IBP 2 Capnography Integrated Thermal Printer Nellcor Oximax Sp02 Pulse Oximetry Biphasic Defibrillation, Pacing
D500 Defibrillator/Monitor LCD Waveform & Text display NIBP Temperature 1 Temperature 2 IBP 1 IBP 2 Capnography Integrated Thermal Printer Nellcor Oximax Sp02 Pulse Oximetry Biphasic Defibrillation, Pacing
Rolyan Splint Pan OPERATION MANUAL. Item # Small Item # Large
 Rolyan Splint Pan OPERATION MANUAL Item #081544816 - Small Item #081544808 Large PLEASE READ THIS ENTIRE MANUAL BEFORE OPERATING YOUR NEW SPLINT PAN. Failure to follow these instructions could result in
Rolyan Splint Pan OPERATION MANUAL Item #081544816 - Small Item #081544808 Large PLEASE READ THIS ENTIRE MANUAL BEFORE OPERATING YOUR NEW SPLINT PAN. Failure to follow these instructions could result in
Guidance and Declaration - Electromagnetic Compatibility (EMC) for the Delfi PTS ii Portable Tourniquet System
 Guidance and Declaration - Electromagnetic Compatibility (EMC) for the Delfi TS ii ortable Tourniquet System Guidance and manufacturer s declaration electromagnetic emissions The TS ii ortable Tourniquet
Guidance and Declaration - Electromagnetic Compatibility (EMC) for the Delfi TS ii ortable Tourniquet System Guidance and manufacturer s declaration electromagnetic emissions The TS ii ortable Tourniquet
SPAC265-8W. AC-DC power supply module. Features. Description. Applications
 AC-DC power supply module Datasheet production data Features Open frame switch mode power supply European input voltage range Single output 5 V, 8 W peak power, 4 W continuous operating mode EMC compliance
AC-DC power supply module Datasheet production data Features Open frame switch mode power supply European input voltage range Single output 5 V, 8 W peak power, 4 W continuous operating mode EMC compliance
(c) Medisave UK. Notice. Edition 1. i-pad Operator s Manual
 Edition 1 Notice i-pad Operator s Manual CU Medical Systems, Inc. reserves the right to make changes on the device specifications contained in this manual at any time without prior notice or obligation
Edition 1 Notice i-pad Operator s Manual CU Medical Systems, Inc. reserves the right to make changes on the device specifications contained in this manual at any time without prior notice or obligation
SAVI SCOUT Surgical Guidance System. Console Operation Manual
 SAVI SCOUT Surgical Guidance System Console Operation Manual 2 Copyrights and Trademarks 2016 Cianna Medical, Inc. All rights reserved. Patents pending. Cianna Medical and SAVI are registered trademarks
SAVI SCOUT Surgical Guidance System Console Operation Manual 2 Copyrights and Trademarks 2016 Cianna Medical, Inc. All rights reserved. Patents pending. Cianna Medical and SAVI are registered trademarks
WRIST BLOOD PRESSURE MONITOR
 WRIST BLOOD PRESSURE MONITOR Instruction Manual MODEL: ABP801 www.accumed.com TABLE OF CONTENTS INTRODUCTION... 1 NOTES ON SAFETY... 1 ABOUT BLOOD PRESSURE... 3 PRECAUTIONS BEFORE US... 4 FEATURES OF THE
WRIST BLOOD PRESSURE MONITOR Instruction Manual MODEL: ABP801 www.accumed.com TABLE OF CONTENTS INTRODUCTION... 1 NOTES ON SAFETY... 1 ABOUT BLOOD PRESSURE... 3 PRECAUTIONS BEFORE US... 4 FEATURES OF THE
EG medlab. Three Lead ECG OEM board. Version Technical Manual. Medlab GmbH Three Lead ECG OEM Module EG01010 User Manual
 Medlab GmbH Three Lead ECG OEM Module EG01010 User Manual medlab Three Lead ECG OEM board EG01010 Technical Manual Copyright Medlab 2008-2016 Version 1.03 1 Version 1.03 28.04.2016 Medlab GmbH Three Lead
Medlab GmbH Three Lead ECG OEM Module EG01010 User Manual medlab Three Lead ECG OEM board EG01010 Technical Manual Copyright Medlab 2008-2016 Version 1.03 1 Version 1.03 28.04.2016 Medlab GmbH Three Lead
Nursing Beds with Dewert drive system
 Nursing Beds with Dewert drive system GB Casa Med Classic 4 / Classic (FS) Casa Med Ultra / Ultra (FS) Casa Med Classic Low Casa Med Classic (FS) 4 / Classic / Casa FS Med / Casa Classic Med Low Ultra
Nursing Beds with Dewert drive system GB Casa Med Classic 4 / Classic (FS) Casa Med Ultra / Ultra (FS) Casa Med Classic Low Casa Med Classic (FS) 4 / Classic / Casa FS Med / Casa Classic Med Low Ultra
Onyx Vantage 9590 Finger Pulse Oximeter
 %SpO 2 0123 Instructions for Use Onyx Vantage 9590 Finger Pulse Oximeter Indications for Use The Nonin Onyx Vantage 9590 Finger Pulse Oximeter is a small, lightweight, portable device indicated for use
%SpO 2 0123 Instructions for Use Onyx Vantage 9590 Finger Pulse Oximeter Indications for Use The Nonin Onyx Vantage 9590 Finger Pulse Oximeter is a small, lightweight, portable device indicated for use
#
 INSTALLATION MANUAL 2 Contents Getting To Know Your AVANT POLAR HIT TM... 4 Setting up the System... 6 Software Installation... 7 Driver Installation Windows 7... 10 Driver Installation Windows XP... 13
INSTALLATION MANUAL 2 Contents Getting To Know Your AVANT POLAR HIT TM... 4 Setting up the System... 6 Software Installation... 7 Driver Installation Windows 7... 10 Driver Installation Windows XP... 13
GE Healthcare. Dash 2500 The standard of excellence for sub-acuity monitoring
 GE Healthcare Dash 2500 The standard of excellence for sub-acuity monitoring The clinical With monitored patients, even small measurement variations can indicate significant changes in your patient s condition.
GE Healthcare Dash 2500 The standard of excellence for sub-acuity monitoring The clinical With monitored patients, even small measurement variations can indicate significant changes in your patient s condition.
TH008F Multi-function Infrared Forehead Thermometer
 TH008F Multi-function Infrared Forehead Thermometer Specifications Functions Temperature measurement range: Forehead mode: 34~42.2 C (93.2~108 F), Surface mode: -22~80 C (-7.6~176 F) Operating temperature
TH008F Multi-function Infrared Forehead Thermometer Specifications Functions Temperature measurement range: Forehead mode: 34~42.2 C (93.2~108 F), Surface mode: -22~80 C (-7.6~176 F) Operating temperature
MedRx Avant Polar HIT AH-I-MPHITS-5 Effective 11/07/11
 INSTALLATION MANUAL 2 Contents Getting To Know Your AVANT POLAR HIT TM... 4 Setting up the System... 6 Software Installation... 7 Driver Installation Windows 7... 10 Driver Installation Windows XP... 13
INSTALLATION MANUAL 2 Contents Getting To Know Your AVANT POLAR HIT TM... 4 Setting up the System... 6 Software Installation... 7 Driver Installation Windows 7... 10 Driver Installation Windows XP... 13
GTIN:
 GTIN: 00894912002920 TABLE OF CONTENTS Introduction...1 Features...1 Indications for Use...1 Contraindications...1 Warnings & Precautions...2 Controls...3 Operation...4 Recharging the Battery...5 Replacing
GTIN: 00894912002920 TABLE OF CONTENTS Introduction...1 Features...1 Indications for Use...1 Contraindications...1 Warnings & Precautions...2 Controls...3 Operation...4 Recharging the Battery...5 Replacing
#0086.
 INSTALLATION MANUAL Contents Getting to Know Your AVANT REM Speech+... 3 Software Installation... 4 Driver Installation Windows 7... 7 EMC Precautions... 11 Safety... 15 Limited Warranty... 18 #0086 www.medrx-usa.com
INSTALLATION MANUAL Contents Getting to Know Your AVANT REM Speech+... 3 Software Installation... 4 Driver Installation Windows 7... 7 EMC Precautions... 11 Safety... 15 Limited Warranty... 18 #0086 www.medrx-usa.com
IEC Second Edition
 Electromagnetic Compatibility of Medical Electrical Equipment Second Edition Prepared by Mr. James Conrad Presented by Dr. William A. Radasky 1 Second Edition Updates first edition on standards developed
Electromagnetic Compatibility of Medical Electrical Equipment Second Edition Prepared by Mr. James Conrad Presented by Dr. William A. Radasky 1 Second Edition Updates first edition on standards developed
USER MANUAL MHS-2500I. Please take time to read these instructions before starting to use the scale. Version /17
 USER MANUAL MHS-2500I Please take time to read these instructions before starting to use the scale Version 1.0 05/17 Contents Introduction 3 Product Specification 3 Safety Instructions 4 Explanation of
USER MANUAL MHS-2500I Please take time to read these instructions before starting to use the scale Version 1.0 05/17 Contents Introduction 3 Product Specification 3 Safety Instructions 4 Explanation of
INSTRUCTIONS FOR YOUR NEW PLASMAFLOW. Vascular Therapy System (Compressible Limb Sleeve Device)
 INSTRUCTIONS FOR YOUR NEW PLASMAFLOW Vascular Therapy System (Compressible Limb Sleeve Device) Customer Service Toll Free: 888-508-0712 Email: CustomerService@manamed.net Web: www.manamed.net 1511 W. Alton
INSTRUCTIONS FOR YOUR NEW PLASMAFLOW Vascular Therapy System (Compressible Limb Sleeve Device) Customer Service Toll Free: 888-508-0712 Email: CustomerService@manamed.net Web: www.manamed.net 1511 W. Alton
Emprint Ablation System with Thermosphere Technology
 Emprint Ablation System with Thermosphere Technology Specification Guide + + + = FIELD CONTROL THERMAL CONTROL WAVELENGTH CONTROL THERMOSPHERE TM TECHNOLOGY Product Information REF / SKU Product Length
Emprint Ablation System with Thermosphere Technology Specification Guide + + + = FIELD CONTROL THERMAL CONTROL WAVELENGTH CONTROL THERMOSPHERE TM TECHNOLOGY Product Information REF / SKU Product Length
Dash 3000, 4000 & 5000
 GE Healthcare Dash 3000, 4000 & 5000 Flexible acuity monitoring Bedside flexibility and adaptability The Dash monitoring family is a portable monitoring system that is flexible and easy to use. The adaptability
GE Healthcare Dash 3000, 4000 & 5000 Flexible acuity monitoring Bedside flexibility and adaptability The Dash monitoring family is a portable monitoring system that is flexible and easy to use. The adaptability
CardioScreen 1000 Device Manual
 CardioScreen 1000 Device Manual 0197 Tel. +49-36 77 46 29 0 info@medis-de.com Medizinische Messtechnik GmbH Fax +49-36 77 46 29 29 http://www.medis-de.com W.-v.-Siemens-Str. 8, D-98693 Ilmenau Germany
CardioScreen 1000 Device Manual 0197 Tel. +49-36 77 46 29 0 info@medis-de.com Medizinische Messtechnik GmbH Fax +49-36 77 46 29 29 http://www.medis-de.com W.-v.-Siemens-Str. 8, D-98693 Ilmenau Germany
Electromagnetic compatibility Guidance and manufacturer s declaration DIN EN :2007 (IEC :2007)
 Compressor set Equipment Under Test (EUT) Type 028 Type 047 Type 052 Type 085 Electromagnetic compatibility Guidance and manufacturer s declaration DIN EN 60601-1-2:2007 (IEC 60601-1-2:2007) 2017 PARI
Compressor set Equipment Under Test (EUT) Type 028 Type 047 Type 052 Type 085 Electromagnetic compatibility Guidance and manufacturer s declaration DIN EN 60601-1-2:2007 (IEC 60601-1-2:2007) 2017 PARI
User instructions for the ABI-system 100
 User instructions for the ABI-system 100 0124 User instructions for the ABI-system 100 1 ABI-system 100 2 CA04 cuffs (for upper arms) 2 CL04 cuffs (for legs) 1 ABI software CD 1 USB cable 1 boso mains
User instructions for the ABI-system 100 0124 User instructions for the ABI-system 100 1 ABI-system 100 2 CA04 cuffs (for upper arms) 2 CL04 cuffs (for legs) 1 ABI software CD 1 USB cable 1 boso mains
HeRO duet
 HeRO duet CUSTOMER SERVICE TABLE OF CONTENTS TABLE OF CONTENTS OVERVIEW OVERVIEW OVERVIEW OVERVIEW USING HeRO duet USING HeRO duet USING HeRO duet Current HeRO Score HeRO USING HeRO duet USING HeRO duet
HeRO duet CUSTOMER SERVICE TABLE OF CONTENTS TABLE OF CONTENTS OVERVIEW OVERVIEW OVERVIEW OVERVIEW USING HeRO duet USING HeRO duet USING HeRO duet Current HeRO Score HeRO USING HeRO duet USING HeRO duet
Manual Supplement. This supplement contains information necessary to ensure the accuracy of the above manual.
 Manual Title: 5502E Getting Started Supplement Issue: 3 Part Number: 4155211 Issue Date: 9/18 Print Date: November 2012 Page Count: 12 Revision/Date: This supplement contains information necessary to ensure
Manual Title: 5502E Getting Started Supplement Issue: 3 Part Number: 4155211 Issue Date: 9/18 Print Date: November 2012 Page Count: 12 Revision/Date: This supplement contains information necessary to ensure
DOPPLEX VASCULAR DOPPLERS. ...with people in mind
 DOPPLEX VASCULAR DOPPLERS...with people in mind VASCULAR DOPPLEX DOPPLERS Based on over 20 years experience in this field, the latest generation of the world renowned Dopplex handheld doppler range offers
DOPPLEX VASCULAR DOPPLERS...with people in mind VASCULAR DOPPLEX DOPPLERS Based on over 20 years experience in this field, the latest generation of the world renowned Dopplex handheld doppler range offers
Medlab GmbH EG04000 User Manual. medlab. Four Lead ECG OEM board EG Technical Manual. Copyright Medlab Version Version 1.
 Medlab GmbH EG04000 User Manual medlab Four Lead ECG OEM board EG04000 Technical Manual Copyright Medlab 2014 1 Medlab GmbH EG04000 User Manual Medlab medizinische Diagnosegeräte GmbH Helmholtzstrasse
Medlab GmbH EG04000 User Manual medlab Four Lead ECG OEM board EG04000 Technical Manual Copyright Medlab 2014 1 Medlab GmbH EG04000 User Manual Medlab medizinische Diagnosegeräte GmbH Helmholtzstrasse
IDEAL INDUSTRIES, INC. TECHNICAL MANUAL MODEL: MODEL: Multimeter Service Information
 IDEAL INDUSTRIES, INC. TECHNICAL MANUAL MODEL: 61-340 MODEL: 61-342 Multimeter Service Information The Service Information provides the following information: Precautions and safety information Specifications
IDEAL INDUSTRIES, INC. TECHNICAL MANUAL MODEL: 61-340 MODEL: 61-342 Multimeter Service Information The Service Information provides the following information: Precautions and safety information Specifications
Manual Supplement. This supplement contains information necessary to ensure the accuracy of the above manual.
 Manual Title: 550A Getting Started Supplement Issue: Part Number: 415509 Issue Date: 9/18 Print Date: November 01 Page Count: 19 Revision/Date: This supplement contains information necessary to ensure
Manual Title: 550A Getting Started Supplement Issue: Part Number: 415509 Issue Date: 9/18 Print Date: November 01 Page Count: 19 Revision/Date: This supplement contains information necessary to ensure
The following languages can be found on our website: French. German. Spanish. Italian. Swedish. Portuguese. Russian. Dutch.
 Manufactured By: 5580 S. Nogales Hwy. Tucson, Az 85706 USA Telephone: 800-975-7987 Fax: 520-294-6061 www.westmedinc.com PN 74586, Rev. 10 MT Promedt Consulting GmbH Altenhofstr. 80 66386 St. Ingbert, Germany
Manufactured By: 5580 S. Nogales Hwy. Tucson, Az 85706 USA Telephone: 800-975-7987 Fax: 520-294-6061 www.westmedinc.com PN 74586, Rev. 10 MT Promedt Consulting GmbH Altenhofstr. 80 66386 St. Ingbert, Germany
User's Manual. LEICKE Sharon Blood Pressure Monitor with Bluetooth. Product number: LH67402
 User's Manual LEICKE Sharon Blood Pressure Monitor with Bluetooth Product number: LH67402 Thank you for purchasing the Blood Pressure Monitor from LEICKE Sharon. We continuously work on the development
User's Manual LEICKE Sharon Blood Pressure Monitor with Bluetooth Product number: LH67402 Thank you for purchasing the Blood Pressure Monitor from LEICKE Sharon. We continuously work on the development
DENTAL X-RAY OPERATOR'S INSTRUCTIONS. (for USA) Wall Mount Type...WK
 M 505 DENTAL X-RAY OPERATOR'S INSTRUCTIONS (for USA) Wall Mount Type...WK WARNING This X-ray equipment may be dangerous to patient and operator unless safe exposure factors, operating instructions and
M 505 DENTAL X-RAY OPERATOR'S INSTRUCTIONS (for USA) Wall Mount Type...WK WARNING This X-ray equipment may be dangerous to patient and operator unless safe exposure factors, operating instructions and
SJM MRI Activator. Handheld Device. User's Manual. Model EX4000
 SJM MRI Activator Handheld Device User's Manual Model EX4000 Unless otherwise noted, indicates that the name is a trademark of, or licensed to, St. Jude Medical or one of its subsidiaries. ST. JUDE MEDICAL
SJM MRI Activator Handheld Device User's Manual Model EX4000 Unless otherwise noted, indicates that the name is a trademark of, or licensed to, St. Jude Medical or one of its subsidiaries. ST. JUDE MEDICAL
Operating Manual Infrared thermometer
 Operating Manual Infrared thermometer Model:IT-121 Professional Fast Accurate 1 CONTENTS 1 I n t r o d u c t i o n................ 3 1.1Product intended use 3 2 Basic principle 3 3 Pr o d u c t f e a t
Operating Manual Infrared thermometer Model:IT-121 Professional Fast Accurate 1 CONTENTS 1 I n t r o d u c t i o n................ 3 1.1Product intended use 3 2 Basic principle 3 3 Pr o d u c t f e a t
Neo Ultrasound Module Manual
 Neo Ultrasound Module Manual Installation Instructions For complete User Operating Instructions, including Cautions, Warnings, Dangers, Indications, and Contraindications, refer to the User s Manuals.
Neo Ultrasound Module Manual Installation Instructions For complete User Operating Instructions, including Cautions, Warnings, Dangers, Indications, and Contraindications, refer to the User s Manuals.
REVISION
 Refa User Manual 92-0121-0001-0-3 REVISION 3 2017 0344 TABLE OF CONTENTS 1 SERVICE AND SUPPORT 4 1.1 About this manual 4 1.2 Contact information TMSi 4 1.3 Warranty information 4 2 SAFETY INFORMATION
Refa User Manual 92-0121-0001-0-3 REVISION 3 2017 0344 TABLE OF CONTENTS 1 SERVICE AND SUPPORT 4 1.1 About this manual 4 1.2 Contact information TMSi 4 1.3 Warranty information 4 2 SAFETY INFORMATION
MHz FUNCTION GENERATOR INSTRUCTION MANUAL
 72-6859 20MHz FUNCTION GENERATOR INSTRUCTION MANUAL Table of Contents Introduction 2 Specification 2 EMC 5 Safety 4 Installation 5 Operation 7 Maintenance 8 www.tenma.com 1 Introduction This instrument
72-6859 20MHz FUNCTION GENERATOR INSTRUCTION MANUAL Table of Contents Introduction 2 Specification 2 EMC 5 Safety 4 Installation 5 Operation 7 Maintenance 8 www.tenma.com 1 Introduction This instrument
9 Specifications. Specifications NOMINAL CHARACTERISTICS
 9 Specifications Specifications NOMINAL CHARACTERISTICS WARRANTED CHARACTERISTICS Nominal characteristics describe parameters and attributes that are guaranteed by design, but do not have associated tolerances.
9 Specifications Specifications NOMINAL CHARACTERISTICS WARRANTED CHARACTERISTICS Nominal characteristics describe parameters and attributes that are guaranteed by design, but do not have associated tolerances.
Nerve stimulator TWISTER
 Nerve stimulator TWISTER INSTRUCTIONS FOR USE Softwareversion 1.19 Manufacturer: Dr. Langer Medical GmbH Fabrik Sonntag, Haus 4a 79183 Waldkirch Germany Table of contents 1 Intended use... 5 2 Common
Nerve stimulator TWISTER INSTRUCTIONS FOR USE Softwareversion 1.19 Manufacturer: Dr. Langer Medical GmbH Fabrik Sonntag, Haus 4a 79183 Waldkirch Germany Table of contents 1 Intended use... 5 2 Common
EMC standards. Presented by: Karim Loukil & Kaïs Siala
 Training Course on Conformity and Interoperability on Type Approval testing for Mobile Terminals, Homologation Procedures and Market Surveillance, Tunis-Tunisia, from 20 to 24 April 2015 EMC standards
Training Course on Conformity and Interoperability on Type Approval testing for Mobile Terminals, Homologation Procedures and Market Surveillance, Tunis-Tunisia, from 20 to 24 April 2015 EMC standards
VN415/VO425 Specifications
 VN415/VO425 Specifications Item No. 8105231-1 01/2014 Contents VN415 Specifications... 1 1.1 Technical Standards:...1 1.2 System Requirements:...1 1.3 Included and Optional Parts...2 VO425 Specifications...
VN415/VO425 Specifications Item No. 8105231-1 01/2014 Contents VN415 Specifications... 1 1.1 Technical Standards:...1 1.2 System Requirements:...1 1.3 Included and Optional Parts...2 VO425 Specifications...
Overview of EMC Regulations and Testing. Prof. Tzong-Lin Wu Department of Electrical Engineering National Taiwan University
 Overview of EMC Regulations and Testing Prof. Tzong-Lin Wu Department of Electrical Engineering National Taiwan University What is EMC Electro-Magnetic Compatibility ( 電磁相容 ) EMC EMI (Interference) Conducted
Overview of EMC Regulations and Testing Prof. Tzong-Lin Wu Department of Electrical Engineering National Taiwan University What is EMC Electro-Magnetic Compatibility ( 電磁相容 ) EMC EMI (Interference) Conducted
At the core of your critical care emergency response
 At the core of your critical care emergency response Effi cia DFM100 defi brillator/monitor To deliver high levels of care, you need to make quick, informed decisions at the scene of an emergency and across
At the core of your critical care emergency response Effi cia DFM100 defi brillator/monitor To deliver high levels of care, you need to make quick, informed decisions at the scene of an emergency and across
C B. Models. Models 2MOPP
 AC/DC Medical Power Supplies High power density 3" x 5" open frame medical power supply 450 Watt with forced air cooling 320 Watt convection cooled without derating up to 50 C Medical certification to
AC/DC Medical Power Supplies High power density 3" x 5" open frame medical power supply 450 Watt with forced air cooling 320 Watt convection cooled without derating up to 50 C Medical certification to
TEST REPORT... 1 CONTENT...
 CONTENT TEST REPORT... 1 CONTENT... 2 1 TEST RESULTS SUMMARY... 3 2 EMC RESULTS CONCLUSION... 4 3 LABORATORY MEASUREMENTS... 6 4 EMI TEST... 7 4.1 CONTINUOUS CONDUCTED DISTURBANCE VOLTAGE TEST... 7 4.2
CONTENT TEST REPORT... 1 CONTENT... 2 1 TEST RESULTS SUMMARY... 3 2 EMC RESULTS CONCLUSION... 4 3 LABORATORY MEASUREMENTS... 6 4 EMI TEST... 7 4.1 CONTINUOUS CONDUCTED DISTURBANCE VOLTAGE TEST... 7 4.2
National Voluntary Laboratory Accreditation Program
 National Voluntary Laboratory Accreditation Program SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 Element Materials Technology Elbridge 4939 Jordan Road Elbridge, NY 13060 Mrs. Vicki Albertson Phone: 503-844-4066
National Voluntary Laboratory Accreditation Program SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 Element Materials Technology Elbridge 4939 Jordan Road Elbridge, NY 13060 Mrs. Vicki Albertson Phone: 503-844-4066
Instructions for Use English
 0123 Instructions for Use English Onyx II Model 9560 Finger Pulse Oximeter Indications for Use The Nonin Onyx II Model 9560 Finger Pulse Oximeter is a small, lightweight, portable, wireless device indicated
0123 Instructions for Use English Onyx II Model 9560 Finger Pulse Oximeter Indications for Use The Nonin Onyx II Model 9560 Finger Pulse Oximeter is a small, lightweight, portable, wireless device indicated
Harmonic Current emission EN :2014 Class A Pass. Voltage Fluctuation and Flicker EN :2013 Clause 5 Pass
 Reference No.: WTS15F0323845E Page 2 of 33 1 Test Summary Test Item Mains Terminal Disturbance Voltage, 148.5kHz to 30MHz Disturbance Power, 30MHz to 300MHz Discontinuous Disturbance (Click) Radiated Emission,
Reference No.: WTS15F0323845E Page 2 of 33 1 Test Summary Test Item Mains Terminal Disturbance Voltage, 148.5kHz to 30MHz Disturbance Power, 30MHz to 300MHz Discontinuous Disturbance (Click) Radiated Emission,
INSTALLATION MANUAL AVANT HIT+ Hearing Instrument Test Chamber.
 INSTALLATION MANUAL AVANT Hearing Instrument Test Chamber HIT+ www.medrx-int.com Contents Getting To Know Your AVANT HIT+. 3 Computer Requirements 4 Setup System. 5 Software Installation.. 6 Sound Card
INSTALLATION MANUAL AVANT Hearing Instrument Test Chamber HIT+ www.medrx-int.com Contents Getting To Know Your AVANT HIT+. 3 Computer Requirements 4 Setup System. 5 Software Installation.. 6 Sound Card
Product Name: COMPER SMART DOPPLER FETAL MONITOR. Product Model: DFMX
 DFMX User Manual Document Num: History of Change: User Manual JS02114DFMX V1.0 Issue Date: Version: Editor: Reviewer: Approver: Date:xx-xx-xx Date:xx-xx-xx Date:xx-xx-xx Product Name: COMPER SMART DOPPLER
DFMX User Manual Document Num: History of Change: User Manual JS02114DFMX V1.0 Issue Date: Version: Editor: Reviewer: Approver: Date:xx-xx-xx Date:xx-xx-xx Date:xx-xx-xx Product Name: COMPER SMART DOPPLER
For the electronic measurement of current: DC, AC, pulsed..., with galvanic separation between the primary and the secondary circuit.
 Current Transducer IN 1000-S N = 1000 A For the electronic measurement of current: DC, AC, pulsed..., with galvanic separation between the primary and the secondary circuit. Features Closed loop (compensated)
Current Transducer IN 1000-S N = 1000 A For the electronic measurement of current: DC, AC, pulsed..., with galvanic separation between the primary and the secondary circuit. Features Closed loop (compensated)
For ultra-high precision measurement of current: DC, AC, pulsed..., with galvanic separation between primary and secondary. Applications.
 Current Transducer IT 200-S ULTRASTAB I PM = 200 A For ultra-high precision measurement of current: DC, AC, pulsed..., with galvanic separation between primary and secondary. Features Closed loop (compensated)
Current Transducer IT 200-S ULTRASTAB I PM = 200 A For ultra-high precision measurement of current: DC, AC, pulsed..., with galvanic separation between primary and secondary. Features Closed loop (compensated)
Ceiling Model Ceiling Model for LEDview Plus and Heliodent Plus. Installation Requirements. New as of: English
 New as of: 03.2017 Ceiling Model Ceiling Model for LEDview Plus and Heliodent Plus Installation Requirements English Installation Requirements Ceiling Version = Dentsply Sirona Installation Requirements
New as of: 03.2017 Ceiling Model Ceiling Model for LEDview Plus and Heliodent Plus Installation Requirements English Installation Requirements Ceiling Version = Dentsply Sirona Installation Requirements
OPERATING MANUAL MINIDOP ES-100VX POCKET DOPPLER
 OPERATING MANUAL MINIDOP ES-100VX POCKET DOPPLER CONTENTS * Features.......................... 1 * Cautions.......................... 2 * Clinical applications................. 4 * Operating controls..................
OPERATING MANUAL MINIDOP ES-100VX POCKET DOPPLER CONTENTS * Features.......................... 1 * Cautions.......................... 2 * Clinical applications................. 4 * Operating controls..................
A Comparison Between MIL-STD and Commercial EMC Requirements Part 2. By Vincent W. Greb President, EMC Integrity, Inc.
 A Comparison Between MIL-STD and Commercial EMC Requirements Part 2 By Vincent W. Greb President, EMC Integrity, Inc. OVERVIEW Compare and contrast military (i.e., MIL-STD) and commercial EMC immunity
A Comparison Between MIL-STD and Commercial EMC Requirements Part 2 By Vincent W. Greb President, EMC Integrity, Inc. OVERVIEW Compare and contrast military (i.e., MIL-STD) and commercial EMC immunity
For ultra-high precision measurement of current: DC, AC, pulsed..., with galvanic separation between primary and secondary. Applications.
 Current Transducer IT 205-S ULTRASTAB I PN = 200 A For ultra-high precision measurement of current: DC, AC, pulsed..., with galvanic separation between primary and secondary. Features Wide operating temperature
Current Transducer IT 205-S ULTRASTAB I PN = 200 A For ultra-high precision measurement of current: DC, AC, pulsed..., with galvanic separation between primary and secondary. Features Wide operating temperature
2620 Modular Measurement and Control System
 European Union (EU) Council Directive 89/336/EEC Electromagnetic Compatibility (EMC) Test Report 2620 Modular Measurement and Control System Sensoray March 31, 2006 April 4, 2006 Tests Conducted by: ElectroMagnetic
European Union (EU) Council Directive 89/336/EEC Electromagnetic Compatibility (EMC) Test Report 2620 Modular Measurement and Control System Sensoray March 31, 2006 April 4, 2006 Tests Conducted by: ElectroMagnetic
JB3000. Operation Manual
 JB3000 HANDHELD PULSE OXIMETER Operation Manual Important: Do not operate the John Bunn JB3000 Handheld Pulse Oximeter without first reading and understanding this manual. Save this manual for future use.
JB3000 HANDHELD PULSE OXIMETER Operation Manual Important: Do not operate the John Bunn JB3000 Handheld Pulse Oximeter without first reading and understanding this manual. Save this manual for future use.
User Instruction Computer Assisted Local Analgesia. 337 Marion, Le Gardeur QC, Canada, J5Z 4W8
 User Instruction Computer Assisted Local Analgesia 1-800-667-9622 337 Marion, Le Gardeur QC, Canada, J5Z 4W8 USER INSTRUCTION Congratulations on your new CALAJECT! Please read these instructions thoroughly
User Instruction Computer Assisted Local Analgesia 1-800-667-9622 337 Marion, Le Gardeur QC, Canada, J5Z 4W8 USER INSTRUCTION Congratulations on your new CALAJECT! Please read these instructions thoroughly
Appendix A: Specifications
 Established 1981 Advanced Test Equipment Rentals www.atecorp.com 800-404-ATEC (2832) Appendix A: Specifications This section provides a complete description of the video measurement set specifications.
Established 1981 Advanced Test Equipment Rentals www.atecorp.com 800-404-ATEC (2832) Appendix A: Specifications This section provides a complete description of the video measurement set specifications.
TPP 40 Series, 40 Watt. Order code Output voltage Output current max. Efficiency. max. screw terminal pin connector Vout 1 Vout 2 Vout 3
 , Features High power density 40 W power supply (enclosed / open frame) 2 x MOPP Medical safety according to AAMI/ANSI ES 60601-1:2005(R) and IEC/EN 60601-1 3rd edition Ready to meet ErP directive, < 0.3
, Features High power density 40 W power supply (enclosed / open frame) 2 x MOPP Medical safety according to AAMI/ANSI ES 60601-1:2005(R) and IEC/EN 60601-1 3rd edition Ready to meet ErP directive, < 0.3
MASIMO RADICAL 7 Signal Extraction Pulse CO-Oximeter
 MASIMO RADICAL 7 Signal Extraction Pulse CO-Oximeter Women s Health Manual MCH Only Policy Group: Cardiovascular Approved by: Heather Crosland Director, Women s Health, Covenant Health, GNH/MCH Site Lead
MASIMO RADICAL 7 Signal Extraction Pulse CO-Oximeter Women s Health Manual MCH Only Policy Group: Cardiovascular Approved by: Heather Crosland Director, Women s Health, Covenant Health, GNH/MCH Site Lead
EVOLV TM Upper Arm Blood Pressure Monitor
 INSTRUCTION MANUAL EVOLV TM Upper Arm Blood Pressure Monitor Model BP7000 ENGLISH TABLE OF CONTENTS Before using the monitor INTRODUCTION...4 INTENDED USE...5 IMPORTANT SAFETY INFORMATION...6 Operating
INSTRUCTION MANUAL EVOLV TM Upper Arm Blood Pressure Monitor Model BP7000 ENGLISH TABLE OF CONTENTS Before using the monitor INTRODUCTION...4 INTENDED USE...5 IMPORTANT SAFETY INFORMATION...6 Operating
For ultra-high precision measurement of current: DC, AC, pulsed..., with galvanic separation between primary and secondary. Applications.
 Current Transducer IT 605-S ULTRASTAB I PN = 600 A For ultra-high precision measurement of current: DC, AC, pulsed..., with galvanic separation between primary and secondary. Features Wide operating temperature
Current Transducer IT 605-S ULTRASTAB I PN = 600 A For ultra-high precision measurement of current: DC, AC, pulsed..., with galvanic separation between primary and secondary. Features Wide operating temperature
Discontinuous Disturbance (Click) EN :2006+A1:2009+A2:2011 Clause N/A** Radiated Emission, 30MHz to 1000MHz
 Reference No.: WTN13F0706038E Page 2 of 40 1 Test Summary Test Item Mains Terminal Disturbance Voltage, 148.5kHz to 30MHz Disturbance Power, 30MHz to 300MHz EMISSION Test Standard Class / Severity Result
Reference No.: WTN13F0706038E Page 2 of 40 1 Test Summary Test Item Mains Terminal Disturbance Voltage, 148.5kHz to 30MHz Disturbance Power, 30MHz to 300MHz EMISSION Test Standard Class / Severity Result
SPEAKER SC-COBRA-PEEK-WP
 2403 260 00132 SPEAKER-1115-3.5-SC-COBRA-PEEK-WP The 11 15 3.5 mm COBRA-PEEK-WP is an advanced miniature speaker of rectangular shape, specifically is a high end miniature speaker specifically designed
2403 260 00132 SPEAKER-1115-3.5-SC-COBRA-PEEK-WP The 11 15 3.5 mm COBRA-PEEK-WP is an advanced miniature speaker of rectangular shape, specifically is a high end miniature speaker specifically designed
Vista 120 Patient Monitoring Solution
 Vista 120 Patient Monitoring Solution Hospitals have a common challenge: provide the best care possible in an environment where patient populations are growing, financial constraints are increasing and
Vista 120 Patient Monitoring Solution Hospitals have a common challenge: provide the best care possible in an environment where patient populations are growing, financial constraints are increasing and
ECG & Respiration Transmitter LX Operation Manual
 ECG & Respiration Transmitter LX-7120 Operation Manual Before using this device, read this operation manual thoroughly. Keep this manual near the device for future reference. Federal Law restricts this
ECG & Respiration Transmitter LX-7120 Operation Manual Before using this device, read this operation manual thoroughly. Keep this manual near the device for future reference. Federal Law restricts this
*Notebook is excluded
 Biomedical Measurement Training System This equipment is designed for students to learn how to design specific measuring circuits and detect the basic physiological signals with practical operation. Moreover,
Biomedical Measurement Training System This equipment is designed for students to learn how to design specific measuring circuits and detect the basic physiological signals with practical operation. Moreover,
User Guide Guide d utilisation Guía del usuario Guia do Utilizador
 MODEL 9570 White dashed line do NOT print User Guide Guide d utilisation Guía del usuario Guia do Utilizador Spine 6963-001-04 Cover.indd 2 5/24/2010 2:20:39 PM Table of Contents Introduction...1 Contents
MODEL 9570 White dashed line do NOT print User Guide Guide d utilisation Guía del usuario Guia do Utilizador Spine 6963-001-04 Cover.indd 2 5/24/2010 2:20:39 PM Table of Contents Introduction...1 Contents
Toshiba Model 3000 With options T-A or T-B
 Toshiba Model 3000 With options T-A or T-B OPERATION MANUAL Designed exclusively to operate with Toshiba Medical Systems CT Scanners Cardiac Trigger Monitor 2010 IVY Biomedical Systems Inc. All Rights
Toshiba Model 3000 With options T-A or T-B OPERATION MANUAL Designed exclusively to operate with Toshiba Medical Systems CT Scanners Cardiac Trigger Monitor 2010 IVY Biomedical Systems Inc. All Rights
GAMMA 20 DIGITAL MULTIMETER
 Gamma 20 www.sifamtinsley.co.uk DATASHEET Issue 1.0 Multifunction Meters Transducers & Isolators Temperature Controllers Converters & Recorders Digital Panel Meters Current Transformers Analogue Panel
Gamma 20 www.sifamtinsley.co.uk DATASHEET Issue 1.0 Multifunction Meters Transducers & Isolators Temperature Controllers Converters & Recorders Digital Panel Meters Current Transformers Analogue Panel
MINISCAV Vacuum Pump Manual
 MINISCAV Vacuum Pump Manual North American Model Caution: Federal law restricts this device to sale by or on the order of a physician or dentist. MR Unsafe - Do not place or use Miniscav in or near an
MINISCAV Vacuum Pump Manual North American Model Caution: Federal law restricts this device to sale by or on the order of a physician or dentist. MR Unsafe - Do not place or use Miniscav in or near an
Product Specification sheet
 INPUT MINIMUM RATED MAXIMUM Input Voltage 90V AC 100 240V AC 264V AC Input Frequency 47 Hz 50 / 60 Hz 63 Hz Input Current * 350mA Inrush Current **
INPUT MINIMUM RATED MAXIMUM Input Voltage 90V AC 100 240V AC 264V AC Input Frequency 47 Hz 50 / 60 Hz 63 Hz Input Current * 350mA Inrush Current **
For the electronic measurement of current: DC, AC, pulsed..., with galvanic separation between the primary and the secondary circuit.
 Current Transducer IN 1000-S I P N = 1000 A For the electronic measurement of current: DC, AC, pulsed..., with galvanic separation between the primary and the secondary circuit. Features Closed loop (compensated)
Current Transducer IN 1000-S I P N = 1000 A For the electronic measurement of current: DC, AC, pulsed..., with galvanic separation between the primary and the secondary circuit. Features Closed loop (compensated)
This annex is valid from: to Replaces annex dated: Locations where activities are performed under accreditation
 Annex to declaration accreditation (scope accreditation) Locations where activities are performed under accreditation Location Abbreviation/ location code Head Location Vijzelmolenlaan 5 & 7 3447 GX oerden
Annex to declaration accreditation (scope accreditation) Locations where activities are performed under accreditation Location Abbreviation/ location code Head Location Vijzelmolenlaan 5 & 7 3447 GX oerden
Sonicator Plus 994 Specifications
 Sonicator Plus 994 Specifications General Specifications: Input: Certification: Domestic model ETL and C-ETL Listed: Domestic model Classification: CE model Year 2000 Compliant Weight: Dimensions: Operating
Sonicator Plus 994 Specifications General Specifications: Input: Certification: Domestic model ETL and C-ETL Listed: Domestic model Classification: CE model Year 2000 Compliant Weight: Dimensions: Operating
Technical data ESA614 Electrical Safety Analyzer
 Technical data ESA614 Electrical Safety Analyzer The ESA614 Electrical Safety Analyzer brings fast and simple automated testing in the form of a portable analyzer to healthcare technology professionals
Technical data ESA614 Electrical Safety Analyzer The ESA614 Electrical Safety Analyzer brings fast and simple automated testing in the form of a portable analyzer to healthcare technology professionals
User instructions. boso ABI-system 100
 User instructions boso ABI-system 100 0124 Contents 1 Contents of package 4 2 Minimum system requirements 4 3 Explanation of symbols and operating controls 5 4 Explanation of icons 6 5 Preliminary remarks
User instructions boso ABI-system 100 0124 Contents 1 Contents of package 4 2 Minimum system requirements 4 3 Explanation of symbols and operating controls 5 4 Explanation of icons 6 5 Preliminary remarks
DC/DC power module 1.8 V / 5A / 9W
 PKF 4918 B I DC/DC power module 1.8 V / 5A / 9W SMD package with ultra low component height 8.0 mm (0.315 in.) 80% efficiency at full load 1,500 Vdc isolation voltage Synchronous rectification MTTF >10
PKF 4918 B I DC/DC power module 1.8 V / 5A / 9W SMD package with ultra low component height 8.0 mm (0.315 in.) 80% efficiency at full load 1,500 Vdc isolation voltage Synchronous rectification MTTF >10
Voltage Isolator 4 (T9405MA)
 Voltage Isolator 4 (T9405MA) Installation and User Guide Thought Technology Ltd. 2180 Belgrave Avenue, Montreal, QC H4A 2L8 Canada Tel: (800) 361-3651 ۰ (514) 489-8251 Fax: (514) 489-8255 E-mail: mail@thoughttechnology.com
Voltage Isolator 4 (T9405MA) Installation and User Guide Thought Technology Ltd. 2180 Belgrave Avenue, Montreal, QC H4A 2L8 Canada Tel: (800) 361-3651 ۰ (514) 489-8251 Fax: (514) 489-8255 E-mail: mail@thoughttechnology.com
