MEMBER COMPANIES AND PARTICIPATING AUTHORITIES
|
|
|
- Asher Ralph Nelson
- 6 years ago
- Views:
Transcription
1 MISSION To maintain a leadership role in identifying and applying scientific principles for the purpose of advancing regulatory and HTA policies and processes in developing and facilitating access to medicinal products. Key Activities International Workshops: Meetings for members are convened at which invited participant interactions are optimised to facilitate networking, constructive discussion, recommendations and actions. CIRS Research Projects: Specialised research and surveys are carried out among leading pharmaceutical companies and regulatory and HTA agencies with expert analyses and interpretation of the findings. Identification of and Advocacy for Best International Practices: Using findings from our Workshops and research projects CIRS interacts with companies, regulators, HTA agencies and other international organisations to promulgate efficiencies in global medicine development. THE CIRS 2018 AGENDA Publications and Presentations: Reports are prepared from Workshops and projects. Dissemination of findings and recommendations through the R&D Briefing series, conference presentations, papers in peer-reviewed journals and the CIRS website are key aspects of the CIRS educational communication mission. MEMBER COMPANIES AND PARTICIPATING AUTHORITIES Member Companies USA Europe Japan AbbVie AstraZeneca Astellas Amgen Bayer Eisai Biogen GlaxoSmithKline Takeda Celgene Idorsia Eli Lilly and Co. Merck KgaA/ EMD Serono Johnson & Johnson Novartis Merck & Co Novo Nordisk Pfizer Roche Shire Sanofi Vertex Servier UCB HTA and Coverage Bodies Country Organisation Australia Belgium Brazil Canada Croatia Denmark England, Wales Europe France Finland Italy Lithuania Norway Poland Portugal Scotland Spain Sweden Switzerland The Netherlands United States PBAC INAMI; KCE CONITEC CADTH; DSEN, Canadian Institutes of Health Research, INESSS, Alberta Health Services AAZ Danish Health and Medicines Authority NICE EUnetHTA HAS THL AIFA VASPVT NOKC AHTAPol INFARMED Scottish Medicines Consortium CAHIAQ, Osteba TLV BAG ZIN UnitedHealth Group; TEC, Blue Cross/Blue Shield Association; Kaiser Permanente Institute for Health Policy; AHRQ; OPTUM Participating Regulatory Authorities Country Authority Argentina ANMAT Australia TGA Brazil ANVISA Canada Health Canada Chile ANAMED China SFDA; CDE Chinese Taipei TFDA; CDE Colombia INVIMA EU EMA India CDSCO Indonesia NAFDC Israel MoH Japan MHLW, PMDA Jordan JFDA Kuwait KDFC Malaysia NCPB Mexico COFEPRIS Oman MoH Peru DIGEMID Philippines DOH, FDA Qatar SCH Saudi Arabia SFDA Singapore HSA South Africa MCC/SAHPRA South Korea MFDS Sweden MPA Switzerland Swissmedic Turkey TITCK United Arab Emirates MoH United Kingdom MHRA United States FDA Scientific Advisory Council Chair: Prof Sir Alasdair Breckenridge, Professor Clinical Pharmacology, University of Liverpool; Former Chairman, MHRA, UK Vice-Chair: Adjunct Prof John Skerritt, Deputy Secretary for Health Products Regulation, Department of Health, Canberra, Australia Dr Petra Dörr, Deputy Executive Director, Swissmedic Prof Hans-Georg Eichler, Senior Medical Officer, EMA Dr Ian Hudson, Chief Executive, MHRA, UK Dr John Lim, Executive Director of CoRE; Chairman of the Singapore Clinical Research Institute. Senior Advisor, Singapore Ministry of Health; Professor of Practice, Duke-NUS Medical School and NUS Saw Swee Hock School of Public Health. Dr Brian O Rourke, CEO and President CADTH, Canada Dr Tomas Salmonson, Chair, CHMP/EMA Dr Jarbas Barbosa da Silva Júnior, Diretor-Presidente, Agência Nacional de Vigilância Sanitária (ANVISA) Dr Fabio Bisordi, Global Head International Regulatory Policy, F.Hoffmann-La Roche Ltd Dr Jay T. Backstrom, SVP, Regulatory Affairs and Pharmacovigilance, Celgene Corporation Dr Tim Garnett, CMO, SVP, Eli Lilly Adrian Griffin, Vice President for HTA Policy Johnson & Johnson Dr Paul Huckle, Chief Regulatory Officer and SVP, GlaxoSmithKline Dr David Jefferys, SVP, Head of Global Regulatory, Eisai Europe Ltd Dr Ron Robison, VP, RQS Regulatory Affairs, R&D QA, and Patient Safety, AbbVie Dr Joseph Scheeren, Head of Global Regulatory Affairs, Bayer Healthcare Company Ltd Pam Smith, VP, Europe and Emerging Markets Regulatory Affairs, Astra Zeneca Dr Mary Baker, Past President, European Brain Council, UK Dr Murray Lumpkin, Senior Fellow, Bill and Melinda Gates Foundation Prof Stuart Walker, Founder, CIRS
2 DRIVING THEMES BENEFITS OF MEMBERSHIP 2018 represents the culmination of a seven-year plan directed by the Scientific Advisory Council to more fully align CIRS activities in the regulatory and access arenas. Because of CIRS special ability to coordinate the input and activities of multiple stakeholders from a global perspective, the new Regulatory and Access Programme will, starting in 2018, address our activities in this holistic manner. Membership to the Regulatory and Access Programme is open to all pharmaceutical companies, in particular those engaged in research and development of new active substances, prescription medicines, devices and biologics with a view to global product development, regulatory affairs and market access. The benefits enjoyed by members of CIRS include: Be part of the small interactive CIRS Workshops, which provide exceptional learning and networking opportunities where you can interact with peers from industry, regulatory authorities, HTA agencies and academia in an atmosphere of informed and productive discussion Full registration and accommodation (excluding travel) for two participants at each Workshop The opportunity to meet and network with senior regulatory personnel from government agencies, international pharmaceutical companies and academia The ability to contribute to the direction of the programme of work for CIRS and put forward subjects for discussion and debate at future Workshops as well as topics for surveys and studies The opportunity to be nominated for participation in the Advisory Management Committee or the Scientific Advisory Council, Steering Committees and Taskforces Exclusive, priority access to - Information derived from studies and surveys to which your organisation has contributed - Reports and slide presentations from CIRS Workshops Early access to - Reports and supportive documents from all Workshops and projects, projects highlighting regulatory and HTA developments, issues and attitudes as a unique information resource - Archives of all CIRS publications including survey and Workshop reports and R&D briefings The fee for the 2018 Regulatory and Access Programme entitles member organisations to all of the benefits of membership described in this brochure; this includes the full registration and accommodation (excluding travel) for two participants at each Workshop and registration for one person to each of the annual Forums. Additional participants may attend Workshops (space permitting) and will be assessed a registration fee ( 950 per person per Workshop plus VAT where applicable), to cover direct participation costs (conference rate, meals and accommodations, administration and overhead; travel excluded).
3 2018 WORKSHOPS 7-8 March, Johannesburg, South Africa Practical implementation of reliance models: What are the barriers and facilitators to successful application of these models for innovative medicines, generics and variations Understand how to practically implement reliance models for decision making in the review of medicines, variations and generics and how agencies/consortia can overcome implementation hurdles and focus on the benefits of utilising these approaches Recommend practical and acceptable reliance models for evaluating new medicines, variations and generics and how to ensure the success of these as approaches to decision making that allow agencies to focus on value-added activities and provide timely patient availability to good quality medicines that are safe and effective When can and should reliance models be used (by design or default) and what data and trust need to be in place to enable their effective and efficient use? Identify which strategies, systems and technologies may provide the evidence agencies will be willing to consider to meet the growing demand for a life cycle approach to medicines evaluation Recommend how post-approval evaluations need to develop to ensure that they are fit for purpose to meet the evidence demands of different stakeholders How can post-approval evaluations be used to generate not just safety information but better information around effectiveness and value of treatment? June, Tysons Corner, Virginia, USA Advancing the on-market evaluation of medicines: Evolving post-approval assessments for a life cycle approach to medicine evaluation Identify which types of early upstream interactions or partnering are considered able to provide the right environment for developing innovative medicines September, Surrey, UK Enabling innovation Early upstream partnering to enhance downstream innovation and decision making Recommend ways in which early upstream partnering can enhance downstream innovation and what developments are necessary to ensure that early interactions are fit for purpose to meet the demands of different stakeholders How can companies and regulatory and HTA agencies utilise early interactions to enable the development and review of new innovative medicines?
4 The 2018 Programme of Work: Evolution of the deliverables Global Development Programme Track 2 paid registrations per Workshop (3 WS) Global Development (regulatory focused) Technical Forum (annual) Regulatory advocacy with ICH+ countries Targeted international regulatory advocacy Support for the Annual Regulator s Forum For HTA Track New Combined Regulatory and Access Programme 1 paid registration per Workshop (3 WS) Two paid registrations per Workshop European, Canadian and US HTA advocacy (3 WS per year) -- Regulatory-focussed Technical Forum (annual) registration fee included (accommodation not included) Aligned regulatory and access advocacy with ICH+ countries -- HTA Focused Technical Forum (annual) HTA/HEOR-focussed Technical Forum (annual) - registration fee included (accommodation not included) Advocacy with access agencies in the global environment Aligned global international advocacy across regulatory and access agencies -- Support for the Annual Regulator s Forum; ad hoc Agency Discussion Meetings; new periodic HTA agency webinars -- Semi-annual HTA teleconferences Semi-annual teleconferences (2 regulatory focus and 2 HTA/HEOR focus) Focus Study participation Focus Study participation Focus Study participation across regulatory and access topics Regulatory agency performance metrics benchmarking Key regulatory projects: BR, isabre, PhD student support Communications: Global R&D Briefings; publications; meeting presentations Key HTA projects: Factors influencing HTA recommendations in Europe; Exploring Approaches to HEOR/HTA decision making; Commonality in evidentiary requirement across regulatory and HTA stakeholders --- Access to Regulatory and Reimbursement Atlas Communications: Global R&D Briefings; publications; meeting presentations -- Regulatory agency performance metrics benchmarking; HTADock integrated regulatory and HTA database outcomes analyses Key sligned projects Regulatory: isabre Aligned projects: Quality Scorecards/Decision Making activities; Facilitated regulatory and access pathways; Commonality in evidentiary requirement across regulatory and HTA stakeholders PhD student support- regulatory and HTA thesis themes Access to Regulatory and Reimbursement Atlas Communications: Advance access to Global R&D Briefings; meeting presentations; workshops slides through members only site; CIRSpotlight app to disseminate publicly available communications Company Insight Seminars Company Insight Seminars Company Insight Seminars (focused on regulatory and access as requested) Regional Insight Seminars for affiliates -- Aligned Regional Insight Seminars for affiliates
5 CIRS - The Centre for Innovation in Regulatory Science - is a neutral, independent UK-based subsidiary company, forming part of Clarivate Analytics. The mission of CIRS is to maintain a leadership role in identifying and applying scientific principles for the purpose of advancing regulatory and HTA policies and processes. CIRS provides an international forum for industry, regulators, HTA and other healthcare stakeholders to meet, debate and develop regulatory and reimbursement policy through the innovative application of regulatory science. It is governed and operated for the sole support of its members activities. The organisation has its own dedicated management and advisory boards, and its funding is derived from membership dues, related activities, special projects and grants. Centre for Innovation in Regulatory Science (CIRS) Friars House 160 Blackfriars Road London SE1 8EZ UK cirs@cirsci.org Website: Lawrence Liberti, Executive Director Neil McAuslane, Director Prisha Patel, Manager, Global Development Programme Tina Wang, Manager, HTA Programme Gill Hepton, Administrator LLiberti@cirsci.org nmcauslane@cirsci.org ppatel@cirsci.org twang@cirsci.org ghepton@cirsci.org V7 NoF (25jan2018)
Aligning global value-based decision making THE CIRS 2018 AGENDA CONSENSUS TRUST ACCESS
 Aligning global value-based decision making THE CIRS 2018 AGENDA CONSENSUS TRUST ACCESS MISSION To maintain a leadership role in identifying and applying scientific principles for the purpose of advancing
Aligning global value-based decision making THE CIRS 2018 AGENDA CONSENSUS TRUST ACCESS MISSION To maintain a leadership role in identifying and applying scientific principles for the purpose of advancing
Country Organisation. CADTH; DSEN, Canadian Institutes of Health Research, INESSS, Alberta Health Services. Danish Health and Medicines Authority
 MISSION To maintain a leadership role in identifying and applying scientific principles for the purpose of advancing regulatory and HTA policies and processes in developing and facilitating access to medicinal
MISSION To maintain a leadership role in identifying and applying scientific principles for the purpose of advancing regulatory and HTA policies and processes in developing and facilitating access to medicinal
Aligning global value-based decision making THE CIRS 2019 AGENDA CONSENSUS TRUST ACCESS
 Aligning global value-based decision making THE CIRS 2019 AGENDA CONSENSUS TRUST ACCESS MISSION To maintain a leadership role in identifying and applying scientific principles for the purpose of advancing
Aligning global value-based decision making THE CIRS 2019 AGENDA CONSENSUS TRUST ACCESS MISSION To maintain a leadership role in identifying and applying scientific principles for the purpose of advancing
24-25 January 2013 PROGRAMME. Intercontinental Financial Street, Beijing, P.R. China
 Regulatory Review How do agencies ensure the quality of the decision? The role of decision frameworks in the review of new medicines: What are the challenges and solutions that can facilitate agencies
Regulatory Review How do agencies ensure the quality of the decision? The role of decision frameworks in the review of new medicines: What are the challenges and solutions that can facilitate agencies
Workshop on. Evolving the Regulatory Review Process What are the features which enable a transparent, timely, predictable and good quality review?
 Workshop on Evolving the Regulatory Review Process What are the features which enable a transparent, timely, predictable and good quality review? 6 7 December 2011 PROGRAMME Intercontinental Hotel, Kuala
Workshop on Evolving the Regulatory Review Process What are the features which enable a transparent, timely, predictable and good quality review? 6 7 December 2011 PROGRAMME Intercontinental Hotel, Kuala
Building quality into HTA and Coverage Decision- Making Processes: What are the features of good practice in HTA?
 Workshop on Building quality into HTA and Coverage Decision- Making Processes: What are the features of good practice in HTA? 2 nd & 3 rd December 2013 Sheraton Heathrow Airport Hotel, UK PROGRAMME Organisers:
Workshop on Building quality into HTA and Coverage Decision- Making Processes: What are the features of good practice in HTA? 2 nd & 3 rd December 2013 Sheraton Heathrow Airport Hotel, UK PROGRAMME Organisers:
Annual Benefit-Risk Workshop
 Annual Benefit-Risk Workshop Implementing an Internationally Acceptable Framework for the Benefit- Risk Assessment of Medicines: How close are we to this objective? 20-21 June 2013 PROGRAMME Venue: The
Annual Benefit-Risk Workshop Implementing an Internationally Acceptable Framework for the Benefit- Risk Assessment of Medicines: How close are we to this objective? 20-21 June 2013 PROGRAMME Venue: The
Finn Børlum Kristensen, MD, PhD Director, EUnetHTA Secretariat Danish Health and Medicines Authority (EUnetHTA Coordinator) Copenhagen, Denmark
 EUnetHTA European network for Health Technology Assessment DATABASES, REGISTRIES AND OTHER DATA CAPTURE TOOLS: HOW CAN WE AVOID MULTIPLICITY AND CREATE AN INTEGRATED DATA-CAPTURE APPROACH ALONG THE PRODUCT
EUnetHTA European network for Health Technology Assessment DATABASES, REGISTRIES AND OTHER DATA CAPTURE TOOLS: HOW CAN WE AVOID MULTIPLICITY AND CREATE AN INTEGRATED DATA-CAPTURE APPROACH ALONG THE PRODUCT
The Assessment of Benefits and Harms and Their Relative Importance for Patients, Industry and Agencies: How should they be captured?
 The Assessment of Benefits and Harms and Their Relative Importance for Patients, Industry and Agencies: How should they be captured? 2-3 April 2014 PROGRAMME Woodlands Park Hotel, Cobham, Surrey, UK CENTRE
The Assessment of Benefits and Harms and Their Relative Importance for Patients, Industry and Agencies: How should they be captured? 2-3 April 2014 PROGRAMME Woodlands Park Hotel, Cobham, Surrey, UK CENTRE
Current Status and Challenges of Bilateral/Multilateral Meetings
 Current Status and Challenges of Bilateral/Multilateral Meetings Junko Sato, PhD International Liaison Officer PMDA 26th Annual EuroMeeting 25-27 March 2014 ACV, Vienna Austria Disclaimer The views and
Current Status and Challenges of Bilateral/Multilateral Meetings Junko Sato, PhD International Liaison Officer PMDA 26th Annual EuroMeeting 25-27 March 2014 ACV, Vienna Austria Disclaimer The views and
Gary Condran Associate Director Bureau of Pharmaceutical Sciences, Therapeutic Product Directorate, HPFB, Health Canada
 Gary Condran Associate Director Bureau of Pharmaceutical Sciences, Therapeutic Product Directorate, HPFB, Health Canada EDQM International Conference 19-20 September 2017 1 Concept History Mission Objectives
Gary Condran Associate Director Bureau of Pharmaceutical Sciences, Therapeutic Product Directorate, HPFB, Health Canada EDQM International Conference 19-20 September 2017 1 Concept History Mission Objectives
Mapping of HTA in Europe " Regulatory and Reimbursement Atlas"
 CIRS- Centre for Innovation in Regulatory Science 1 CONSENSUS TRUST ACCESS Mapping of HTA in Europe " Regulatory and Reimbursement Atlas" Tina Wang Manager, HTA Programme twang@cirsci.org 20 May 2016 Brussels,
CIRS- Centre for Innovation in Regulatory Science 1 CONSENSUS TRUST ACCESS Mapping of HTA in Europe " Regulatory and Reimbursement Atlas" Tina Wang Manager, HTA Programme twang@cirsci.org 20 May 2016 Brussels,
Table of Contents Executive Summary 29
 Contents Table of Contents Executive Summary 29 Section 1: Introduction 33 Section 2: World 37 2.1.1. Main consumers 37 2.1.2. Main producers 2015 and 2016 39 2.1.3. Main importers 2015 and 2016 40 2.1.4.
Contents Table of Contents Executive Summary 29 Section 1: Introduction 33 Section 2: World 37 2.1.1. Main consumers 37 2.1.2. Main producers 2015 and 2016 39 2.1.3. Main importers 2015 and 2016 40 2.1.4.
Who Reads and Who Follows? What analytics tell us about the audience of academic blogging Chris Prosser Politics in
 Who Reads and Who Follows? What analytics tell us about the audience of academic blogging Chris Prosser Politics in Spires @caprosser 1 What do we want to know about the audience for academic blogging?
Who Reads and Who Follows? What analytics tell us about the audience of academic blogging Chris Prosser Politics in Spires @caprosser 1 What do we want to know about the audience for academic blogging?
Global Board Seats Held by Women ±1 16.1% 15.8% 15.0% 15.0% 14.0% 13.9% 12.7% 11.2% 10.8% 10.8% 10.3% 9.5% 9.3% 9.0% 8.8% 8.7% 8.7% 8.5% 8.4% 7.
 Global Board Seats Held by Women ±1 Equality Mark Norway Sweden Finland United States South Africa Israel United Kingdom Netherlands Denmark France Germany Poland Turkey Canada Ireland Spain Hong Kong
Global Board Seats Held by Women ±1 Equality Mark Norway Sweden Finland United States South Africa Israel United Kingdom Netherlands Denmark France Germany Poland Turkey Canada Ireland Spain Hong Kong
NFC Forum: The Evolution of a Consortium
 NFC Forum: The Evolution of a Consortium Presented by Greg Kohn Sr. Operations Director, NFC Forum ANSI Open Forum: Building Bridges across the Standards Ecosystem October 9, 2012 Part of the World Standards
NFC Forum: The Evolution of a Consortium Presented by Greg Kohn Sr. Operations Director, NFC Forum ANSI Open Forum: Building Bridges across the Standards Ecosystem October 9, 2012 Part of the World Standards
Mapping Your Success 2013 BSI Healthcare Road Show
 Mapping Your Success 2013 BSI Healthcare Road Show Welcome & Outline Objectives for Today Please let me introduce myself.. Gary Slack Global Director BSI Medical Devices Based London 2 Changing Global
Mapping Your Success 2013 BSI Healthcare Road Show Welcome & Outline Objectives for Today Please let me introduce myself.. Gary Slack Global Director BSI Medical Devices Based London 2 Changing Global
THE CORPORATE REPUTATION OF PHARMA THE PATIENT PERSPECTIVE IN 2015 (EASTERN-EUROPE EDITION)
 THE CORPORATE REPUTATION OF PHARMA THE PATIENT PERSPECTIVE IN 2015 (EASTERN-EUROPE EDITION) Feedback from 93 Eastern-European patient groups PUBLISHED JULY 2016 CONTENTS ABOUT THIS REPORT Page 1 WHAT DID
THE CORPORATE REPUTATION OF PHARMA THE PATIENT PERSPECTIVE IN 2015 (EASTERN-EUROPE EDITION) Feedback from 93 Eastern-European patient groups PUBLISHED JULY 2016 CONTENTS ABOUT THIS REPORT Page 1 WHAT DID
PROTECT Project. Joanna Groves Chief Executive Officer 27 April 2010 EFPIA Patient Think-Tank Brussels, Belgium
 PROTECT Project Joanna Groves Chief Executive Officer 27 April 2010 EFPIA Patient Think-Tank Brussels, Belgium Content of Presentation About IAPO Overview of PROTECT Work Package 4 - New methods for data
PROTECT Project Joanna Groves Chief Executive Officer 27 April 2010 EFPIA Patient Think-Tank Brussels, Belgium Content of Presentation About IAPO Overview of PROTECT Work Package 4 - New methods for data
WORKSHOP SYNOPSIS. Building quality into HTA/coverage decision-making processes: What are the features of good practice in HTA?
 Building quality into HTA/coverage decision-making processes: What are the features of good practice in HTA? 2 3 December 2013 HEATHROW, UK WORKSHOP SYNOPSIS Synopsis authors Neil McAuslane, PhD Tina Wang,
Building quality into HTA/coverage decision-making processes: What are the features of good practice in HTA? 2 3 December 2013 HEATHROW, UK WORKSHOP SYNOPSIS Synopsis authors Neil McAuslane, PhD Tina Wang,
Swissmedic, Swiss Agency for Therapeutic Products
 PMDA International Forum, 8 February 2014 Swissmedic, Swiss Agency for Therapeutic Products Jürg H. Schnetzer, Executive Director Grüezi. Bonjour. Buongiorno. Allegra. 2 Overview Swissmedic Introduction
PMDA International Forum, 8 February 2014 Swissmedic, Swiss Agency for Therapeutic Products Jürg H. Schnetzer, Executive Director Grüezi. Bonjour. Buongiorno. Allegra. 2 Overview Swissmedic Introduction
Building the Benefit- Risk Toolbox:
 Building the Benefit- Risk Toolbox: Are there enough common elements across the different methodologies to enable a consensus on a scientifically acceptable framework for making benefit-risk decisions?
Building the Benefit- Risk Toolbox: Are there enough common elements across the different methodologies to enable a consensus on a scientifically acceptable framework for making benefit-risk decisions?
Economic Outlook for 2016
 Economic Outlook for 2016 Arturo Bris Professor of Finance, IMD Director, IMD World Competitiveness Center Yale International Center for Finance European Corporate Governance Institute 2015 IMD International.
Economic Outlook for 2016 Arturo Bris Professor of Finance, IMD Director, IMD World Competitiveness Center Yale International Center for Finance European Corporate Governance Institute 2015 IMD International.
IGDRP Mission, Scope, How it works
 IGDRP Mission, Scope, How it works IGDRP-EDQM Workshop Strasbourg, France 13 May 2016 Dr. Craig Simon Associate Director, Bureau of Pharmaceutical Sciences Therapeutic Products Directorate Health Canada
IGDRP Mission, Scope, How it works IGDRP-EDQM Workshop Strasbourg, France 13 May 2016 Dr. Craig Simon Associate Director, Bureau of Pharmaceutical Sciences Therapeutic Products Directorate Health Canada
Nurturing Talent Reinforcing the Interaction between Research, Innovation and Education
 Nurturing Talent Reinforcing the Interaction between Research, Innovation and Education Henrietta Egerth FFG, Austrian Research Promotion Agency INNOVATING INNOVATION May 18, 2015 Introducation It is the
Nurturing Talent Reinforcing the Interaction between Research, Innovation and Education Henrietta Egerth FFG, Austrian Research Promotion Agency INNOVATING INNOVATION May 18, 2015 Introducation It is the
Frame through-beam sensors
 Frame through-beam sensors Features Wide range of sizes: passage sizes from 25 x 23 mm to 300 x 397.5 mm Metal housings Integrated evaluation unit Connection by means of connector Degree of protection
Frame through-beam sensors Features Wide range of sizes: passage sizes from 25 x 23 mm to 300 x 397.5 mm Metal housings Integrated evaluation unit Connection by means of connector Degree of protection
European Connected Health Alliance Bringing needs and solutions together for the Future of Health. ECHAlliance Update
 European Connected Health Alliance Bringing needs and solutions together for the Future of Health ECHAlliance Update Gregor Cuzak, International Ecosystem Coordinator - Gregor@echalliance.com Damian O
European Connected Health Alliance Bringing needs and solutions together for the Future of Health ECHAlliance Update Gregor Cuzak, International Ecosystem Coordinator - Gregor@echalliance.com Damian O
Integrated Scientific Advice Workshop: ISPOR Glasgow
 Integrated Scientific Advice Workshop: ISPOR Glasgow Early Integrated Scientific Advice in Product Development: Get Real and Adapt to Accelerate Patient Access ICONplc.com Overview At today s patient-centered
Integrated Scientific Advice Workshop: ISPOR Glasgow Early Integrated Scientific Advice in Product Development: Get Real and Adapt to Accelerate Patient Access ICONplc.com Overview At today s patient-centered
Advancing the business of intellectual property globally.
 Advancing the business of intellectual property globally www.lesi.org Advancing the business of intellectual property globally 2 WHAT IS LES? A premier global business association for licensing whose members,
Advancing the business of intellectual property globally www.lesi.org Advancing the business of intellectual property globally 2 WHAT IS LES? A premier global business association for licensing whose members,
DAB+ Digital Radio. Global update. Vasant Venkatramani, WorldDAB IFTV Broadcast Istanbul, November 2018
 DAB+ Digital Radio Global update Vasant Venkatramani, WorldDAB IFTV Broadcast Istanbul, 15-16 November 2018 2 1. Radio needs DAB+ 2. DAB+ around the world 3. DAB+ in the car and home 4. About WorldDAB
DAB+ Digital Radio Global update Vasant Venkatramani, WorldDAB IFTV Broadcast Istanbul, 15-16 November 2018 2 1. Radio needs DAB+ 2. DAB+ around the world 3. DAB+ in the car and home 4. About WorldDAB
GLOBAL PRO BONO REPORT. Law is essential to creating a just society, but law does not create justice by itself.
 20 13 GLOBAL PRO BONO REPORT Law is essential to creating a just society, but law does not create justice by itself. In 2013, PILnet s clearinghouses in Hungary, Russia, and its crossborder Global Clearinghouse
20 13 GLOBAL PRO BONO REPORT Law is essential to creating a just society, but law does not create justice by itself. In 2013, PILnet s clearinghouses in Hungary, Russia, and its crossborder Global Clearinghouse
Remote participation in Question sessions Audio options VoIP
 Remote participation in Question sessions Remote participation will use GoToMeeting. Participants must be registered to the SG13 meeting in der to be able to join 1. Use your laptop s microphone and speakers
Remote participation in Question sessions Remote participation will use GoToMeeting. Participants must be registered to the SG13 meeting in der to be able to join 1. Use your laptop s microphone and speakers
Public Private Partnerships & Idea selection
 www.pwc.nl Public Private Partnerships & Idea selection A tool to select technological healthcare innovation ideas PPPs should select technical healthcare innovation ideas by answering seven questions
www.pwc.nl Public Private Partnerships & Idea selection A tool to select technological healthcare innovation ideas PPPs should select technical healthcare innovation ideas by answering seven questions
stripax The professional stripping tool
 stripax The professional stripping tool stripax the original: developed from experience Weidmüller is the world s leading manufacturer of solutions for electrical connectivity, transmission, conditioning
stripax The professional stripping tool stripax the original: developed from experience Weidmüller is the world s leading manufacturer of solutions for electrical connectivity, transmission, conditioning
EU, USA and Japan (II) Reports from Regulators on Exchange Assignments
 EU, USA and Japan (II) Reports from Regulators on Exchange Assignments Yoshikazu Hayashi MHLW/PMDA Liaison at EMA PMDA 24th Annual EuroMeeting 26-28 March 2012 Copenhagen, Denmark Disclaimer The views
EU, USA and Japan (II) Reports from Regulators on Exchange Assignments Yoshikazu Hayashi MHLW/PMDA Liaison at EMA PMDA 24th Annual EuroMeeting 26-28 March 2012 Copenhagen, Denmark Disclaimer The views
The compact test- disconnect terminal interface system for protection and secondary technology
 POCON POWER Connector The compact test- disconnect terminal interface system for protection and secondary technology POCON the compact test-disconnect terminal interface system Safe control and testing
POCON POWER Connector The compact test- disconnect terminal interface system for protection and secondary technology POCON the compact test-disconnect terminal interface system Safe control and testing
WOODWORKING TECHNOLOGY IN EUROPE: HIGHLIGHTS European Federation of Woodworking Technology Manufacturers
 European Federation of Woodworking Technology Manufacturers ADVANCED ECONOMIES - GDP % GROWTH RATE 2017 8,0 7,0 6,0 5,0 4,0 3,0 2,0 1,0 0,0 Ireland Malta Slovenia Estonia Latvia Czech Republic Cyprus
European Federation of Woodworking Technology Manufacturers ADVANCED ECONOMIES - GDP % GROWTH RATE 2017 8,0 7,0 6,0 5,0 4,0 3,0 2,0 1,0 0,0 Ireland Malta Slovenia Estonia Latvia Czech Republic Cyprus
PO01275C Tabor East Neighborhood Meeting. Monday, April 20, :30 PM 8:30 PM
 PO01275C Tabor East Neighborhood Meeting Monday, April 20, 2015 6:30 PM 8:30 PM 1 Opening Remarks, Introductions, Explanation of Agenda and Procedure Lenny Borer Moderator 2 Portland Office for Community
PO01275C Tabor East Neighborhood Meeting Monday, April 20, 2015 6:30 PM 8:30 PM 1 Opening Remarks, Introductions, Explanation of Agenda and Procedure Lenny Borer Moderator 2 Portland Office for Community
Through-beam ring sensors
 Throughbeam ring sensors Features Wide range of sizes: ring diameters of 10, 15 and 20 mm Metal housings Separate evaluation unit Connection by means of S8 connector Degree of protection IP 63 Adjustable
Throughbeam ring sensors Features Wide range of sizes: ring diameters of 10, 15 and 20 mm Metal housings Separate evaluation unit Connection by means of S8 connector Degree of protection IP 63 Adjustable
2017 POST SHOW REPORT
 2017 POST SHOW REPORT Dates Venue Organiser January 18 Wed - 20 Fri, 2017 Tokyo Big Sight, Japan Reed Exhibitions Japan Ltd. www.robodex.jp/en/ What is RoboDEX? Comprehensive trade show for robots RoboDEX
2017 POST SHOW REPORT Dates Venue Organiser January 18 Wed - 20 Fri, 2017 Tokyo Big Sight, Japan Reed Exhibitions Japan Ltd. www.robodex.jp/en/ What is RoboDEX? Comprehensive trade show for robots RoboDEX
TECHNOLOGY VISION 2017 IN 60 SECONDS
 TECHNOLOGY VISION 2017 IN 60 SECONDS GET THE ESSENTIALS THE BIG READ SHORT ON TIME? VIEW HIGHLIGHTS 5 MIN READ VIEW FULL REPORT 45 MIN READ VIEW SHORT REPORT 15 MIN READ OVERVIEW #TECHV1SION2017 2017 TREND
TECHNOLOGY VISION 2017 IN 60 SECONDS GET THE ESSENTIALS THE BIG READ SHORT ON TIME? VIEW HIGHLIGHTS 5 MIN READ VIEW FULL REPORT 45 MIN READ VIEW SHORT REPORT 15 MIN READ OVERVIEW #TECHV1SION2017 2017 TREND
STAINLESS STEEL STAINLESS STEEL MANUFACTURING STAINLESS STEEL TRADING BRIGHT BARS WIRES PRECISION COMPONENTS
 STAINLESS STEEL BRIGHT BARS WIRES PRECISION COMPONENTS BHANSALI is diversified business group with interests in Stainless Steel, Textiles and ABS Plastics. Under the dynamic leadership of Mr. Pukhraj Bhansali
STAINLESS STEEL BRIGHT BARS WIRES PRECISION COMPONENTS BHANSALI is diversified business group with interests in Stainless Steel, Textiles and ABS Plastics. Under the dynamic leadership of Mr. Pukhraj Bhansali
Science, Technology & Innovation Indicators
 Science, Technology & Innovation Indicators Adnan Badran NASIC Conference cum Workshop on Herbal Drug Development for Socio-economic Uplift in Developing World The University of Jordan, September 6-8,
Science, Technology & Innovation Indicators Adnan Badran NASIC Conference cum Workshop on Herbal Drug Development for Socio-economic Uplift in Developing World The University of Jordan, September 6-8,
Other appointments: Chairman of the Advisory Committee of Zentiva Group, Industrial Advisor EQT.
 Executive Committee Guido Oelkers Chief Executive Officer Born 1965. Employed since 2017. PhD in Strategic Management, University of South Australia, Master of Economics, South Bank University, London,
Executive Committee Guido Oelkers Chief Executive Officer Born 1965. Employed since 2017. PhD in Strategic Management, University of South Australia, Master of Economics, South Bank University, London,
2018/2019 HCT Transition Period OFFICIAL COMPETITION RULES
 2018/2019 HCT Transition Period OFFICIAL COMPETITION RULES 1. INTRODUCTION These HCT Transition Period Official Competition Rules ( Official Rules ) govern how players earn Hearthstone Competitive Points
2018/2019 HCT Transition Period OFFICIAL COMPETITION RULES 1. INTRODUCTION These HCT Transition Period Official Competition Rules ( Official Rules ) govern how players earn Hearthstone Competitive Points
Japan s Leading Exhibition for Robotics Technologies Jan. 17[Wed]-19[Fri], 2018 Tokyo Big Sight, Japan
![Japan s Leading Exhibition for Robotics Technologies Jan. 17[Wed]-19[Fri], 2018 Tokyo Big Sight, Japan Japan s Leading Exhibition for Robotics Technologies Jan. 17[Wed]-19[Fri], 2018 Tokyo Big Sight, Japan](/thumbs/78/76786007.jpg) Japan s Leading Exhibition for Robotics Technologies Jan. 17[Wed]-19[Fri], 2018 Tokyo Big Sight, Japan Web: http://www.robodex.jp/en/ POST SHOW REPORT 2018 FACTS & FIGURES 17,186 Visitors 200 Exhibitors
Japan s Leading Exhibition for Robotics Technologies Jan. 17[Wed]-19[Fri], 2018 Tokyo Big Sight, Japan Web: http://www.robodex.jp/en/ POST SHOW REPORT 2018 FACTS & FIGURES 17,186 Visitors 200 Exhibitors
BenchTop Extraction Arms with unbeatable flexibility
 BenchTop Extraction Arms with unbeatable flexibility A new generation of BenchTop extraction arms with unbeatable flexibility Nederman introduces a new generation of BenchTop arms the FX, FX and FX. These
BenchTop Extraction Arms with unbeatable flexibility A new generation of BenchTop extraction arms with unbeatable flexibility Nederman introduces a new generation of BenchTop arms the FX, FX and FX. These
(3) How does one obtain patent protection?
 Patenting in Kenya (1) Introduction A patent gives the owner the exclusive rights to prevent others from manufacturing, using or selling the protected invention in a given country. A patent is a legally
Patenting in Kenya (1) Introduction A patent gives the owner the exclusive rights to prevent others from manufacturing, using or selling the protected invention in a given country. A patent is a legally
Regulatory status for using RFID in the UHF spectrum 3 May 2006
 Regulatory status for using RFID in the UHF spectrum 3 May NOTE: The following countries were updated since the last publication of 3 March : Thailand, Romania. The table attached provides an overview
Regulatory status for using RFID in the UHF spectrum 3 May NOTE: The following countries were updated since the last publication of 3 March : Thailand, Romania. The table attached provides an overview
INAHTA Working Group Ethical Issues in HTA
 Pre-conference Workshop at HTAi 2006 Working Group Ethical Issues in HTA Pre-conference workshop HTAi 2006 Overview Who is? Why establish a working group on ethics? What has been accomplished? What net?
Pre-conference Workshop at HTAi 2006 Working Group Ethical Issues in HTA Pre-conference workshop HTAi 2006 Overview Who is? Why establish a working group on ethics? What has been accomplished? What net?
Corporate Compliance & Transparency
 4 th Annual Conference Corporate Compliance & Transparency in the Pharmaceutical Industry 24 25 February 2016 Zürich, Switzerland Conference In Numbers 84 Attendees 2 Days 29 Speakers 19 Participating
4 th Annual Conference Corporate Compliance & Transparency in the Pharmaceutical Industry 24 25 February 2016 Zürich, Switzerland Conference In Numbers 84 Attendees 2 Days 29 Speakers 19 Participating
BIOPHARMACEUTICAL SECTOR TRAINING SESSION (DAY ONE) 1 SEPTEMBER 2014
 APEC Business Ethics for SMEs Forum: Promoting Ethical Environments in the Medical Device & Biopharmaceutical Sectors Sofitel Nanjing Zhongshan Nanjing, China 1-3 September 2014 BIOPHARMACEUTICAL SECTOR
APEC Business Ethics for SMEs Forum: Promoting Ethical Environments in the Medical Device & Biopharmaceutical Sectors Sofitel Nanjing Zhongshan Nanjing, China 1-3 September 2014 BIOPHARMACEUTICAL SECTOR
Workshop Guide. Joint Regulators/Industry QbD Workshop January 2014 London, UK. europe.pda.org/ema2014
 EMA together with other EU Health Agencies Joint Regulators/Industry QbD Workshop 28-29 January 2014 London, UK Workshop Guide europe.pda.org/ema2014 ORGANIZED BY PDA EUROPE Letter from the Chairs Dear
EMA together with other EU Health Agencies Joint Regulators/Industry QbD Workshop 28-29 January 2014 London, UK Workshop Guide europe.pda.org/ema2014 ORGANIZED BY PDA EUROPE Letter from the Chairs Dear
BIM Policy Development: Different Countries, Common Approaches
 : Different Countries, Common Approaches Bilal Succar, PhD Director, BIMexcellence.com Mohamad Kassem, PhD Senior Lecturer + Enterprise Fellow, Teesside University 2 this presentation is in Two Parts:
: Different Countries, Common Approaches Bilal Succar, PhD Director, BIMexcellence.com Mohamad Kassem, PhD Senior Lecturer + Enterprise Fellow, Teesside University 2 this presentation is in Two Parts:
GPFI Subgroup: Regulation and Standard-Setting Bodies (SSBs) 2018 Work Plan
 GPFI Subgroup: Regulation and Standard-Setting Bodies (SSBs) 2018 Work Plan Objective of the Subgroup: The 2018 Work Plan of the GPFI Regulation and SSBs Subgroup is organized around the Objectives (Activities)
GPFI Subgroup: Regulation and Standard-Setting Bodies (SSBs) 2018 Work Plan Objective of the Subgroup: The 2018 Work Plan of the GPFI Regulation and SSBs Subgroup is organized around the Objectives (Activities)
Integrate, validate, and implement
 Integrate, validate, and implement Human Identification Professional Services As the world leader in human identification, Thermo Fisher Scientific continues to develop and deliver technologies, services,
Integrate, validate, and implement Human Identification Professional Services As the world leader in human identification, Thermo Fisher Scientific continues to develop and deliver technologies, services,
CNR ICMATE, Padova - Italy. ENEA, Roma - Italy. Monica Fabrizio 1, Amelia Montone 2, Federico Cernuschi 3 1
 Mission Innovation. Clean Energy Materials Innovation Challenge 6: to accelerate the exploration, discovery and use of new high performance, low-cost clean energy materials Monica Fabrizio 1, Amelia Montone
Mission Innovation. Clean Energy Materials Innovation Challenge 6: to accelerate the exploration, discovery and use of new high performance, low-cost clean energy materials Monica Fabrizio 1, Amelia Montone
Opportunities for the pharma supply & support network in Emerging Markets
 Opportunities for the pharma supply & support network in Emerging Markets Parioforma Ltd 55 Princes Gate Exhibition Road South Kensington London SW7 2PN United Kingdom www.parioforma.com Emerging markets
Opportunities for the pharma supply & support network in Emerging Markets Parioforma Ltd 55 Princes Gate Exhibition Road South Kensington London SW7 2PN United Kingdom www.parioforma.com Emerging markets
Brochure More information from
 Brochure More information from http://www.researchandmarkets.com/reports/1342464/ The World Market for Stranded Wire, Cable, Ropes, and Plaited Bands of Iron, Steel, Copper, or Aluminum Excluding Electrically
Brochure More information from http://www.researchandmarkets.com/reports/1342464/ The World Market for Stranded Wire, Cable, Ropes, and Plaited Bands of Iron, Steel, Copper, or Aluminum Excluding Electrically
Getting to Equal, 2016
 Getting to Equal, 2016 Listen. Learn, Lead, 2015 Career Capital, 2014 Defining Success. Your Way, 2013 The Path Forward, 2012 Reinvent Opportunity: Looking Through a New Lens, 2011 Resilience in the Face
Getting to Equal, 2016 Listen. Learn, Lead, 2015 Career Capital, 2014 Defining Success. Your Way, 2013 The Path Forward, 2012 Reinvent Opportunity: Looking Through a New Lens, 2011 Resilience in the Face
48 RobecoSAM The Sustainability Yearbook RobecoSAM Industry Leaders 2016
 48 RobecoSAM The Yearbook 0 RobecoSAM 0 RobecoSAM 0 Company Country Abbott Laboratories health Care Equipment & Supplies united States Agilent Technologies Inc life Sciences Tools & Services united States
48 RobecoSAM The Yearbook 0 RobecoSAM 0 RobecoSAM 0 Company Country Abbott Laboratories health Care Equipment & Supplies united States Agilent Technologies Inc life Sciences Tools & Services united States
CIECA News Letter. Monthly Event Summary. No. 54 / December 2016
 CIECA News Letter No. 54 / December 2016 Monthly Event Summary Luncheon for Members of the Provincial Parliament of Ontario, Canada Delegation At the invitation of CIECA Chairman C.Y. Wang, a delegation
CIECA News Letter No. 54 / December 2016 Monthly Event Summary Luncheon for Members of the Provincial Parliament of Ontario, Canada Delegation At the invitation of CIECA Chairman C.Y. Wang, a delegation
Patented Medicine Prices Review Board P M P R B GUIDELINES REFORM. 15 th Annual Market Access Summit. Douglas Clark Executive Director PMPRB
 Patented Medicine Prices Review Board P M P R B GUIDELINES REFORM Douglas Clark Executive Director PMPRB 15 th Annual Market Access Summit Background Canada enacted a two-fold reform of its drug patent
Patented Medicine Prices Review Board P M P R B GUIDELINES REFORM Douglas Clark Executive Director PMPRB 15 th Annual Market Access Summit Background Canada enacted a two-fold reform of its drug patent
Monthly Summary of Troop Contribution to UN Operations
 Monthly Summary of Troop Contribution to UN Operations Month of Report : 3-Dec-3 Country Description of Post M F Totals ) Albania Individual Police............ 0 Subtotal for Country ) Algeria Experts
Monthly Summary of Troop Contribution to UN Operations Month of Report : 3-Dec-3 Country Description of Post M F Totals ) Albania Individual Police............ 0 Subtotal for Country ) Algeria Experts
WFEO STANDING COMMITTEE ON ENGINEERING FOR INNOVATIVE TECHNOLOGY (WFEO-CEIT) STRATEGIC PLAN ( )
 WFEO STANDING COMMITTEE ON ENGINEERING FOR INNOVATIVE TECHNOLOGY (WFEO-CEIT) STRATEGIC PLAN (2016-2019) Hosted by The China Association for Science and Technology March, 2016 WFEO-CEIT STRATEGIC PLAN (2016-2019)
WFEO STANDING COMMITTEE ON ENGINEERING FOR INNOVATIVE TECHNOLOGY (WFEO-CEIT) STRATEGIC PLAN (2016-2019) Hosted by The China Association for Science and Technology March, 2016 WFEO-CEIT STRATEGIC PLAN (2016-2019)
Treasury and Trade Solutions Citi Commercial Cards. A History of Achievement. A Future of Innovation. May 19-21, 2014
 Treasury and Trade Solutions Citi Commercial Cards A History of Achievement. A Future of Innovation. May 19-21, 2014 Communicating and Marketing Your Program Internally Pauline Smith Carla Vitaliano, The
Treasury and Trade Solutions Citi Commercial Cards A History of Achievement. A Future of Innovation. May 19-21, 2014 Communicating and Marketing Your Program Internally Pauline Smith Carla Vitaliano, The
OECD Science, Technology and Industry Outlook 2008: Highlights
 OECD Science, Technology and Industry Outlook 2008: Highlights Global dynamics in science, technology and innovation Investment in science, technology and innovation has benefited from strong economic
OECD Science, Technology and Industry Outlook 2008: Highlights Global dynamics in science, technology and innovation Investment in science, technology and innovation has benefited from strong economic
The Story of Why. #Wave 7
 The Story of Why #Wave 7 Agenda Welcome to Wave 7 Trends: Social Movements Social is the new normal Trends: Devices The Mobile Revolution is there Cracking The Social Code The search for the why Brands
The Story of Why #Wave 7 Agenda Welcome to Wave 7 Trends: Social Movements Social is the new normal Trends: Devices The Mobile Revolution is there Cracking The Social Code The search for the why Brands
European Network for Health Technology Assessment (EUnetHTA) Joint Action 3
 European Network for Health Technology Assessment (EUnetHTA) Joint Action 3 Zoe Garrett, Senior Technical Adviser Lead WP7 National Implementation and Impact National Institute for Health and Care Excellence
European Network for Health Technology Assessment (EUnetHTA) Joint Action 3 Zoe Garrett, Senior Technical Adviser Lead WP7 National Implementation and Impact National Institute for Health and Care Excellence
Maintaining the Argo bibliographies. Megan Scanderbeg
 Maintaining the Argo bibliographies Megan Scanderbeg AST-17 Meeting in Yokohama, Japan March 216 Update for the past year 39 Argo papers published in 299 days in 215 3 articles in Nature Climate Change
Maintaining the Argo bibliographies Megan Scanderbeg AST-17 Meeting in Yokohama, Japan March 216 Update for the past year 39 Argo papers published in 299 days in 215 3 articles in Nature Climate Change
1204 Reflected Wave Reduction Device
 Instructions 1204 Reflected Wave Reduction Device (Catalog Number 1204-RWR2-09-B, C) This publication will guide you through installation (including mounting, wiring and grounding procedures) of the 1204
Instructions 1204 Reflected Wave Reduction Device (Catalog Number 1204-RWR2-09-B, C) This publication will guide you through installation (including mounting, wiring and grounding procedures) of the 1204
Convergence and Differentiation within the Framework of European Scientific and Technical Cooperation on HTA
 EUnetHTA European network for Health Technology Assessment Convergence and Differentiation within the Framework of European Scientific and Technical Cooperation on HTA University of Tokyo, October 24,
EUnetHTA European network for Health Technology Assessment Convergence and Differentiation within the Framework of European Scientific and Technical Cooperation on HTA University of Tokyo, October 24,
ehealth Ireland Ecosystem members of the ECHAlliance International Ecosystem Network European Ecosystem Network Update
 ehealth Ireland Ecosystem members of the ECHAlliance International Ecosystem Network European Ecosystem Network Update Damian O Connor, Director Ecosystem Development, European Connected Health Alliance
ehealth Ireland Ecosystem members of the ECHAlliance International Ecosystem Network European Ecosystem Network Update Damian O Connor, Director Ecosystem Development, European Connected Health Alliance
INTERNATIONAL CIVIL AVIATION ORGANIZATION
 EUR DOC 024 Attachment INTERNATIONAL CIVIL AVIATION ORGANIZATION EUROPEAN PRINCIPLES AND PROCEDURES FOR THE ALLOCATION OF SECONDARY SURVEILLANCE RADAR MODE S INTERROGATOR CODES (IC) 2011 ATTACHMENT MODE
EUR DOC 024 Attachment INTERNATIONAL CIVIL AVIATION ORGANIZATION EUROPEAN PRINCIPLES AND PROCEDURES FOR THE ALLOCATION OF SECONDARY SURVEILLANCE RADAR MODE S INTERROGATOR CODES (IC) 2011 ATTACHMENT MODE
Automated Frequency Response Measurement with AFG31000, MDO3000 and TekBench Instrument Control Software APPLICATION NOTE
 Automated Frequency Response Measurement with AFG31000, MDO3000 and TekBench Instrument Control Software Introduction For undergraduate students in colleges and universities, frequency response testing
Automated Frequency Response Measurement with AFG31000, MDO3000 and TekBench Instrument Control Software Introduction For undergraduate students in colleges and universities, frequency response testing
Innovation policy mixes and implications on HEIs - emerging conclusions from the OECD innovation policy reviews
 Innovation policy mixes and implications on HEIs - emerging conclusions from the OECD innovation policy reviews Gernot Hutschenreiter Country Studies and Outlook Division Directorate for Science, Technology
Innovation policy mixes and implications on HEIs - emerging conclusions from the OECD innovation policy reviews Gernot Hutschenreiter Country Studies and Outlook Division Directorate for Science, Technology
WIPO Capacity Building Activities and Programs: Activities for Innovation Promotion and Technology Transfer
 WIPO Capacity Building Activities and Programs: Activities for Innovation Promotion and Technology Transfer National Seminar on Intellectual Property (IP) Management and Commercialization of IP Assets
WIPO Capacity Building Activities and Programs: Activities for Innovation Promotion and Technology Transfer National Seminar on Intellectual Property (IP) Management and Commercialization of IP Assets
International Presence, Local Expertise. Introductory Presentation
 International Presence, Local Expertise Introductory Presentation About GlobalM&A Partners Established in 1999 A partnership of 38 leading independent M&A firms Over 200 active dealmakers supported by
International Presence, Local Expertise Introductory Presentation About GlobalM&A Partners Established in 1999 A partnership of 38 leading independent M&A firms Over 200 active dealmakers supported by
PDA 71 Years of Connecting People, Science and Regulation
 PDA 71 Years of Connecting People, Science and Regulation 1 I m happy to be here. Bom Dia. Estou feliz por estar aqui. Richard M. Johnson PDA President & CEO since 2009 38 years experience in US and International
PDA 71 Years of Connecting People, Science and Regulation 1 I m happy to be here. Bom Dia. Estou feliz por estar aqui. Richard M. Johnson PDA President & CEO since 2009 38 years experience in US and International
Biomedical Innovation Has Science Overtaken the System?
 Adaptive Pathways for Transformative Medicinal Products. A New Paradigm with the Enhanced Application of Real-World Evidence? ISPOR 20 th Annual European Congress, Glasgow, Scotland Issue Panel 21, Wednesday,
Adaptive Pathways for Transformative Medicinal Products. A New Paradigm with the Enhanced Application of Real-World Evidence? ISPOR 20 th Annual European Congress, Glasgow, Scotland Issue Panel 21, Wednesday,
Welcome to the IFR Press Conference 30 August 2012, Taipei
 Welcome to the IFR Press Conference 3 August 212, Taipei Continued success of the robotics industry Welcome by IFR President Dr. Shinsuke Sakakibara Presentation of the results of World Robotics 212 Industrial
Welcome to the IFR Press Conference 3 August 212, Taipei Continued success of the robotics industry Welcome by IFR President Dr. Shinsuke Sakakibara Presentation of the results of World Robotics 212 Industrial
Gender Equality Commitment Workshop JUNE 2018
 Gender Equality Commitment Workshop JUNE 2018 Dr. Zsuzsanna Tungli Managing Partner Lorraine Fagan Director Developing Global Leaders/Cultural Training Asia Email ztungli@developinggloballeaders.com lorrainefagan@culturaltrainingasia.com
Gender Equality Commitment Workshop JUNE 2018 Dr. Zsuzsanna Tungli Managing Partner Lorraine Fagan Director Developing Global Leaders/Cultural Training Asia Email ztungli@developinggloballeaders.com lorrainefagan@culturaltrainingasia.com
CDP-EIF ITAtech Equity Platform
 CDP-EIF ITAtech Equity Platform New financial instruments to support technology transfer in Italy TTO Circle Meeting, Oxford June 22nd 2017 June, 2017 ITAtech: the "agent for change" in TT landscape A
CDP-EIF ITAtech Equity Platform New financial instruments to support technology transfer in Italy TTO Circle Meeting, Oxford June 22nd 2017 June, 2017 ITAtech: the "agent for change" in TT landscape A
ISU Symposium The Public Face of Space Strasbourg, France February A quiet and sustainable success story.
 ISU Symposium The Public Face of Space Strasbourg, France 16 18 February 2010 The International Cospas-Sarsat Programme: A quiet and sustainable success story Dany St-Pierre Cospas-Sarsat Secretariat ISU
ISU Symposium The Public Face of Space Strasbourg, France 16 18 February 2010 The International Cospas-Sarsat Programme: A quiet and sustainable success story Dany St-Pierre Cospas-Sarsat Secretariat ISU
CONFERENCE AGENDA. Empowering Your Clinical Trial Operations Through Digital Innovation. 5 6 December 2018, Grand Copthorne Waterfront, Singapore
 CONFERENCE AGENDA Empowering Your Clinical Trial Operations Through Digital Innovation 5 6 December 2018, Grand Copthorne Waterfront, Singapore Researched & Developed by CONFERENCE DAY ONE: WEDNESDAY,
CONFERENCE AGENDA Empowering Your Clinical Trial Operations Through Digital Innovation 5 6 December 2018, Grand Copthorne Waterfront, Singapore Researched & Developed by CONFERENCE DAY ONE: WEDNESDAY,
EUROPE March Brussels, Belgium CALL FOR ABSTRACTS. Join Us at the Crossroads of Healthcare. DIAglobal.org/Europe2020 #DIAEurope2020
 EUROPE 2020 17-19 March Brussels, Belgium CALL FOR ABSTRACTS Join Us at the Crossroads of Healthcare I1 Steering Committee Francis Arickx INAMI, Belgium Matthieu Boudes EPF, Belgium Sini Eskola EFPIA,
EUROPE 2020 17-19 March Brussels, Belgium CALL FOR ABSTRACTS Join Us at the Crossroads of Healthcare I1 Steering Committee Francis Arickx INAMI, Belgium Matthieu Boudes EPF, Belgium Sini Eskola EFPIA,
Telecommunication & Broadcasting Produced by IAR Team Focus Technology Co., Ltd.
 Telecommunication & Broadcasting 2013.6 Produced by IAR Team Focus Technology Co., Ltd. Contents 1. China Telecommunication & Broadcasting Industry Analysis Report from Jan. to March 2013...5 1.1. China
Telecommunication & Broadcasting 2013.6 Produced by IAR Team Focus Technology Co., Ltd. Contents 1. China Telecommunication & Broadcasting Industry Analysis Report from Jan. to March 2013...5 1.1. China
Smart Grid Maturity Model: A Vision for the Future of Smart Grid
 Smart Grid Maturity Model: A Vision for the Future of Smart Grid David W. White Smart Grid Maturity Model Project Manager White is a member of the Resilient Enterprise Management (REM) team in the CERT
Smart Grid Maturity Model: A Vision for the Future of Smart Grid David W. White Smart Grid Maturity Model Project Manager White is a member of the Resilient Enterprise Management (REM) team in the CERT
Innovation and the Changing Practice of Medicine
 Innovation and the Changing Practice of Medicine Japan Medical Innovation Symposium David Epstein, Division Head Novartis Pharmaceuticals Tokyo, August 18, 2015 Agenda The Case for Innovation How Novartis
Innovation and the Changing Practice of Medicine Japan Medical Innovation Symposium David Epstein, Division Head Novartis Pharmaceuticals Tokyo, August 18, 2015 Agenda The Case for Innovation How Novartis
CISCO ONS /100-GHZ INTERLEAVER/DE-INTERLEAVER FOR THE CISCO ONS MULTISERVICE TRANSPORT PLATFORM
 DATA SHEET CISCO ONS 15216 50/100-GHZ INTERLEAVER/DE-INTERLEAVER FOR THE CISCO ONS 15454 MULTISERVICE TRANSPORT PLATFORM The Cisco ONS 15216 50/100-GHz Interleaver/De-interleaver is an advanced 50/100-GHz
DATA SHEET CISCO ONS 15216 50/100-GHZ INTERLEAVER/DE-INTERLEAVER FOR THE CISCO ONS 15454 MULTISERVICE TRANSPORT PLATFORM The Cisco ONS 15216 50/100-GHz Interleaver/De-interleaver is an advanced 50/100-GHz
SZF Associates. SZF is an issues-oriented, evidence-based market research and consulting firm serving the pharmaceutical and biotech industry.
 SZF Associates Company Mission SZF is an issues-oriented, evidence-based market research and consulting firm serving the pharmaceutical and biotech industry. We work with strategic and tactical decision
SZF Associates Company Mission SZF is an issues-oriented, evidence-based market research and consulting firm serving the pharmaceutical and biotech industry. We work with strategic and tactical decision
EBA Master Class The Benefits of International Collaboration. Steve Morgan Co-Chair, EBA Benchmarking Group
 EBA Master Class The Benefits of International Collaboration Steve Morgan Co-Chair, EBA Benchmarking Group Why Collaborate? We all have a common goal - to benefit patients Patients require access to safe
EBA Master Class The Benefits of International Collaboration Steve Morgan Co-Chair, EBA Benchmarking Group Why Collaborate? We all have a common goal - to benefit patients Patients require access to safe
DRAFT. Cardiac Safety Research Consortium CSRC. Membership Committee Charter. 12September2018. Table of Contents
 DRAFT Cardiac Safety Research Consortium CSRC Membership Committee Charter 12September2018 Table of Contents Purpose of the CSRC Membership Charter and CSRC Membership Committee Composition, Responsibilities
DRAFT Cardiac Safety Research Consortium CSRC Membership Committee Charter 12September2018 Table of Contents Purpose of the CSRC Membership Charter and CSRC Membership Committee Composition, Responsibilities
Biometrics and Standardization. Presentation to BioSec 2nd Open Workshop, 19th and 20th January, 2005
 Biometrics and Standardization Presentation to BioSec 2nd Open Workshop, 19th and 20th January, 2005 COPRAS is an FP6 Specific Support Action Project with clear goals Stimulate interaction & exchange between
Biometrics and Standardization Presentation to BioSec 2nd Open Workshop, 19th and 20th January, 2005 COPRAS is an FP6 Specific Support Action Project with clear goals Stimulate interaction & exchange between
Annual report of the Good Manufacturing and Distribution Practice Inspectors Working Group 2017
 17 July 2018 EMA/INS/GMP/19273/2018 Committees and Inspections Annual report of the Good Manufacturing and Distribution Practice Inspectors Working 30 Churchill Place Canary Wharf London E14 5EU United
17 July 2018 EMA/INS/GMP/19273/2018 Committees and Inspections Annual report of the Good Manufacturing and Distribution Practice Inspectors Working 30 Churchill Place Canary Wharf London E14 5EU United
Connecting People, Science and Regulation
 Connecting People, Science and Regulation Bethesda Towers 4350 East West Highway Suite 600 Bethesda, MD 20814 USA Tel: +1 (301) 656-5900 Fax: +1 (301) 986-0296 www.pda.org PDA Europe ggmbh Am Borsigturm
Connecting People, Science and Regulation Bethesda Towers 4350 East West Highway Suite 600 Bethesda, MD 20814 USA Tel: +1 (301) 656-5900 Fax: +1 (301) 986-0296 www.pda.org PDA Europe ggmbh Am Borsigturm
Life Sciences CONFERENCE SERIES. Business Information in a Global Context LIFE SCIENCES IP PORTFOLIO. Business Development Pack
 C5 Business Information in a Global Context Life Sciences CONFERENCE SERIES LIFE SCIENCES IP PORTFOLIO Business Development Pack C5 GROUPS LIFE SCIENCES EVENTS Pharmaceuticals, biotechnology, diagnostics,
C5 Business Information in a Global Context Life Sciences CONFERENCE SERIES LIFE SCIENCES IP PORTFOLIO Business Development Pack C5 GROUPS LIFE SCIENCES EVENTS Pharmaceuticals, biotechnology, diagnostics,
Cisco ONS Metropolitan Dense Wavelength Division Multiplexing 100-GHz FlexLayer Filter Solution
 Data Sheet Cisco ONS 15216 Metropolitan Dense Wavelength Division Multiplexing 100-GHz FlexLayer Filter Solution The Cisco ONS 15216 Metropolitan Dense Wavelength-Division Multiplexing (DWDM) FlexLayer
Data Sheet Cisco ONS 15216 Metropolitan Dense Wavelength Division Multiplexing 100-GHz FlexLayer Filter Solution The Cisco ONS 15216 Metropolitan Dense Wavelength-Division Multiplexing (DWDM) FlexLayer
MSCI GLOBAL MARKET ACCESSIBILITY REVIEW JUNE Competitive landscape
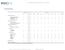 DEVELOPED MARKETS Americas Canada USA Austria Belgium Denmark Finland France Germany Ireland Israel Italy Netherlands Investor qualification requirement ++ ++ ++ ++ ++ ++ ++ ++ ++ ++ ++ ++ Foreign ownership
DEVELOPED MARKETS Americas Canada USA Austria Belgium Denmark Finland France Germany Ireland Israel Italy Netherlands Investor qualification requirement ++ ++ ++ ++ ++ ++ ++ ++ ++ ++ ++ ++ Foreign ownership
Verifying Power Supply Sequencing with an 8-Channel Oscilloscope APPLICATION NOTE
 Verifying Power Supply Sequencing with an 8-Channel Oscilloscope Introduction In systems that rely on multiple power rails, power-on sequencing and power-off sequencing can be critical. If the power supplies
Verifying Power Supply Sequencing with an 8-Channel Oscilloscope Introduction In systems that rely on multiple power rails, power-on sequencing and power-off sequencing can be critical. If the power supplies
