SOP-P094. BioMek 2000 Compound Protocol Cyan/Hypercyt + Analysis
|
|
|
- Kenneth Cox
- 5 years ago
- Views:
Transcription
1 SOP-P094 BioMek 2000 Compound Protocol Cyan/Hypercyt + Analysis Objective: To test serial dilutions of certain compounds Protocol for half plate runs only. One plate of Redox dye and the other plate - JC1 Dye. Automation Plate Layout: 1. Format for a 384 well plate is as follows: a. Columns #3 through #13 have chemical compounds at 9 concentrations, um (300, 100, 33.33, 11.11, 3.70, 1.23, 0.41, 0.137, 0.046) b. Columns #12 through #20 are duplicate sets. c. Method: Drug Dilution_Redox (or JC1) 2. Columns #1,2, are controls: Cells + medium + Dyes, no compounds a. For the Redox plate, use 20ul of 100% Ethanol in row O wells #21 - #24 b. For the JC1 plate, use 2uls of 10mM of Valinomycin in row P wells #21- # Dye Panels: Panel 1, Redox: rows A,C,E,G,I,K,M,O. Panel 2, JC1: rows B,D,F,H,J,L,N,P. a. Duplicates per each dye. b. Method: 30 uls (or 35 uls depending on tips used) of Redox (or JC1) row A (or row B) 4. Cell Concentration: 1x10^7/ml (Approximately 20,000 cells total per well) a. Method: Cells to drugs_redox or JC1 (20uls per well) Assay: BioMek 2000 Robot: 1. On the monitor, Open the Bioworks Icon folder (If missing go to : my computer c:\ Documents and Settings \ All users \ Desktop \ Bioworks) 2. Click on Workstation Server 3. On the back (lower left side) of the Biomek 200, switch on the Robot. 4. Wait until the light on the workstation Server window turns green and is connected with the computer. This takes a few seconds. 5. Once they are connected, just minimize the window. DO NOT CLOSE or it will not be connected. 6. On the Bioworks window, click on the Edit(2) option. 7. Go to Method \ open \ select your method (protocol) you wish to use \ open. 8. If this is the correct method, highlight the first line starting with Pipette \ click on the Run Button (It looks like a man running) 9. Biomek 2000 Configuration Window appears. If correct, Accept All. This will start the procedure. 10. Repeat steps 7 9 each time you need a new Method for the experiment. Page 1 of 9 Document Name: common (M):\SOPS\sopP093.doc May 28, 2013
2 Procedures: 1. Chemicals prepared the same day as assay in a 96 well plate (Method: Drug Dilution) Medium: RMPI % FBSc or NBSc. 3 fold serial dilutions in the medium, 100 ul of 2x concentrations of chemicals. After the medium has been added to the 96 well plate, there will be a pause in the procedure. Add 3 uls in column 3 of the prepared compounds manually. Agitate up and down several times to mix them with the medium. Hit okay to start the drug dilution steps. 2. Transfer chemical + medium to the 384 well plate (Method: Drug Dilution) 3. Add Cells to the 384 well plate - 20uls (Method: Cells to Drugs) 4. Incubate plates for 6 37 C, CO 2 incubator. 5. After incubation time, cover plate with a breathable tape and spin down the rpm, for 5 37 C. 6. Aspirate the supernatant (Depending on what tips you are using, Method: 20ul Tips_Aspr_Row A(B) or Method: Aspir_Supr_Row A(B)_50ul Tips) 7. Gently vortex plate on the vortex in room 122B for a few seconds. This will break up the cell pellet some what before adding the dyes. 8. Use 30uls of dye if you used the 20ul tips for the aspiration step. Use 40uls of dye if you used the 50ul tips for the aspiration step. Method: 30uls (or 40uls) of Redox (or JC1)_Row A(B) Positive Controls: Once the dyes have been added to the plates, you need to add the controls. Redox plate: Add 20uls of 100% Ethanol to row O, wells #21 - #24. JC1 plate: Add 2uls of 10mM Valinomycin to row P, wells # Place in the 37 C incubator for 10 minute incubation time for the dyes. 11. After dye incubation time, shake plates for 12 rpms on the plate shaker in room Cover with breathable tape and spin rpm, for 30 seconds to remove and bubbles. 13. Plates are now ready to run on the Cyan/Hypercyte System. Dyes are made in PBS in 0.1% BSA (SOP-P065 front cover of the BioMek 2000 Binder) Redox Dye Calcein : 30,000 dilution of 1 mg/ml, final 0.03 ug/ml. mbbr: stock 40mM, final 40uM Mitosox: 10mM in DMSO, final 10uM JC1: stock 5mM, final 5uM Page 2 of 9 Document Name: common (M):\SOPS\sopP093.doc May 28, 2013
3 Flow Cytometry Cyan: 1. Instrument must be on 30 minutes prior to receiving samples. 2. Turn on the Monitor (lower right hand corner) / lab password: flow(sp)lab / click arrow 3. Open the Summit 4.3 Icon / click on New / and put the date (yy/month/day: r for Redox Or j for JC1) 4. File / Protocol / Load / click drop down menu / Data (D) / QC / QC_flowcheck_Beads_date 5. Control Panel / Instrument / Startup remove tube from Cyan, close lever / System Clean / window opens (check the amount of cleaning solution, if okay / click yes 6. After the 13 minutes system clean, de-bubble twice. 7. Now you are ready to run the Flowcheck Beads. / open lever / insert tube / close lever / Hit F2 on the key board / set a stop count or just hit F2 again after 5000 events / Record Settings. 8. File / protocol / load / Sky Blue Beads / run beads / Record settings 9. To check the Laser Delay on the Cyan, refer to the SOP-P077 posted next to the Cyan. / Record settings. 10. File / protocol / load / Data (D) / BioMeck 2000 / Redox.plo 11. Run Level II beads for standardize the voltage and gain. Be sure to add the ND filter on the FL1(525) filter and remove the FL2(590) filter completely from the Cyan. (The ND filter is in the plastic tray on the work bench next to the Cyan. It s a small plastic circle with a black marker circle drawn around the edge. Place it in the top opening, press in gently.) 12. Now open / the Redox or JC1 protocol: File / protocol / load / Data D / BioMeck 2000 / Redox.plo or JC1.plo / run the Level II Beads / record settings. You may need to lower or raise the voltage to get the Level II beads as close as possible to the target settings. As of 1/11/20132 Target settings a. Redox: calcein means 160 b. JC1: 525 line means 13 mbbr means line means 224 Mitosox means From the bulk cells remaining, you will need to make the following tubes: Refer to SOP-P095 for tube prep (copy in the front of the BioMek 2000 Binder) a. Cells only, unstained b. Cells + Redox dye (optional: cells + redox + 100% Etoh) Add the dyes, vortex, place c. Cells + JC1 dye in 37 C water bath for a d. Cells + JC1+ Valinomycin 10 minute incubation time. Use these tubes to check the light scatter and to see if the dyes are working Page 3 of 9 Document Name: common (M):\SOPS\sopP093.doc May 28, 2013
4 14. Run the appropriate tubes with the correct protocol. Run the Cells only, unstained for both the Redox and the JC1 protocols. SAVE DATA 15. Under Acquisition tab on the control panel, you will need to save a path way as follows: Save path / my computer / Data (D) / BioMek 2000 / make new folder / date: yy/mo/day / okay Note: Cyan / Hypercyte: See protocol SOP-P070a When the system is completely shut down and needs a complete start up and some troubleshooting. Flow Cytometry Hypercyt: 1. Check the Sheath Tank, Waste Tank and the Cleaning Container. a. Sheath Tank: Contains Millipore Water b. Cleaning Container: Contains 2% Contrad 70 c. Waste Tank: Collects the cells, sheath and the cleaning solution. Note: When the waste container is half way full, undo the quick connects, pour in about ½ cup of bleach and let sit for 24 hours. Take the empty waste container and snap in the quick connects. Take a post-it note labeled Being Bleached and place it on the waste container. After the 24 hours, pour the waste down the sink with running water. (a full waste container is too heavy to lift to dispose of waste) 2. Turn on the Monitors (lower right hand corner) / lab password: flow(sp)lab / click arrow 3. Open both the icons Hypercyt Controller and the Hypercyt Designer 4. Clamp the sample tubing on the peristaltic pump by pushing the white lever on the right closed. Be sure the tubing is half way up the wheel. Buffers needed: They are kept in the mini-fridge in 15cc tubes labeled accordingly. Place them in the provided holder on the Hypercyt. a. Station 1 - PBS + 0.1% BSA: Used for priming and during the shaking of the plate steps. b. Position 3-2.0% Contrad 70 (Company: Decon Labs, Inc, Cat# 1003) : Used in the Clean step c. Position 4 - Sterile, Filtered Millipore water. : Used in the Clean step 5. On the Controller Window, click on the tab prime. This is to check the segment pattern. If it is not a consistent pattern, you can adjust the pressure on the sample tube by turning the black knob behind the wheel. If you still are having a problem, you may have to replace the tubing. Instructions are in the manual. 6. If the segments are looking good, continue priming for 2-3 minutes. Steps to communicate the Hypercyt to the Cyan: a. Click on the Hypercyt SummitAutomater icon b. Set up / Data (D)/ BioMek 2000 / look for the day s date / okay / hit start on the Cyan Monitor / and Reset on the Hypercyt Controller Window. c. The green light on the Hypercyt Controller should come on. If not try again. d. The two soft wares should be communicating now. Page 4 of 9 Document Name: common (M):\SOPS\sopP093.doc May 28, 2013
5 DO NOT CLOSE THE AUTOMATOR BOX, just click anywhere else and it will minimize 7. On the Hypercyt Designer Window, go to New Experiment / BioMek 2000 / new folder put the day s date (yy/mo/day)plus either a r for Redox or j for JC1. First: Select the plate type by the drop down tab / select 384 well plate. Second: In the name space, type r (or j) (YYYYMMDD) / hit create Summarize Proper Labeling - IS VERY IMPORTANT: Experiment name: r (or j) (YYYYMMDD) Plate name: p0001a Analysis name: BioMr (or j) 8. On this window, click on the experiment setup tab / plate model / AMG2_Greiner PPN 384 V-Bottom_40ul 9. Now click the sample settings tab: a. Check Enable Automatic Prime / Duration 30 sec / Cycle up/down b. Check Enable Pre-plate Shake / Duration 30 sec / c. Sample order - Click all rows to be sampled left to right. d. Shake Inter-well Shake / / Probe station S1 / After ever 12 wells / duration 5 secs. e. Flush & Clean Flush Durations for 60 secs. Plate Layouts are shown on the Print Out provided for each dye on the back of this sheet. 10. Once settings are chosen, save experiment. On the Hypercyt Controller window, go to File / open experiment. The one you created will be highlighted / Open. Running Plates: 1. a. Disconnect the tubing on the front of the Cyan. Leave the metal piece connect with the left side tubing. b. Take the Hypercyt sample tubing and connect it to the metal tubing on the Cyan. c. Open lever and put the half plastic tube on. Push it up until you feel it is secure. Close lever. (the half plastic tube is located just to the right in a holder) 2. Prime once again for 1 minute while plate is being shaken on the plate shaker for rpms. Do this step twice. 3. Place the plate on the Hypercyt and hit Run on the Hypercyt Controller window. The Cyan and the Hypercyt will automatically start running. Note: It will take approximately 45 seconds before you see any data on the Cyan Screen. 4. Once the plate is completed, go to Acquisition/save data. Wait until the Cyan has attached the FCS files before doing any analysis. There will we a green check mark in the plate window on the Hypercyt Controller window. See example below. Page 5 of 9 Document Name: common (M):\SOPS\sopP093.doc May 28, 2013
6 Symbol to delete gates Purdue University Cytometry Laboratories Data Analysis: 1. Open HyperView Analysis icon on the Hypercyte monitor. 2. Click new analysis / Browse / Click on the experiment (plate) you want to analyze. (Be sure you are in the correct folder.) / Open 3. In that window there is a name box that says Analysis. Type in BioMr or (j) / Create 4. At the top, be sure the plate number matches the one you want to analyze. a. Go to noise gate tab / change the X axis to SS Lin(10^3) b. Draw a polygonal gate around the whole population, omitting the small amount of noise in the lower left corner. c. Hit Well Finder / left click in the data window / move the wheel on the mouse until all the peaks have gates around them. Gate Symbol Hand symbol to move gates 5. Above the peaks, it will say Well Finder (Noise Gated) 192/192 Wells If not, you will need to look for the missing gate or peak in some situations. 6. To look at the individual wells, do the following steps. a. Click the left single arrow until you see the beginning of the peaks. (either row A for Redox or row B for JC1) b. Now click the right single arrow and look at each peak to see if the gates are on each peak correctly. If not, click on the gate symbol, then Page 6 of 9 Document Name: common (M):\SOPS\sopP093.doc May 28, 2013
7 click on the peak or the empty space where the gate is needed. c. If you need to move a gate, click on the hand symbol and the move the gate where it is needed. 7. When all gates are correct / Hit Save Analysis. 8. Click on the Population tab / Click the Add 2D Histogram icon / change X axis to SS Lin(10^3) / draw a gate around the population / this now becomes Population 1 Gate 9. Click on the Statistics tab. a. Check the Population 1 Box. b. On the parameter menu / check FS Lin Box Mean and FS Lin Box Count (This is to check the accuracy of the counts per well) c. If you want fluorescence, check any of the other parameter boxes. d. Now hit Apply Page 7 of 9 Document Name: common (M):\SOPS\sopP093.doc May 28, 2013
8 Statistics Purdue University Cytometry Laboratories Export FCS Files: 1. File / Export / Export All Well FCS Data 2. Under Population Statistics / Click the Excel Export Box 3. Click the drop down tab / scratch / projects BioMek 2000 / your folder / right click on the Plate name / Rename / control C / in the file name control V / (underscore)_stat / Open folder / SAVE Page 8 of 9 Document Name: common (M):\SOPS\sopP093.doc May 28, 2013
9 Print Name Purdue University Cytometry Laboratories Sign Name Created by: Cheryl Holdman Verified by: Date: 8/2/2013 Page 9 of 9 Document Name: common (M):\SOPS\sopP093.doc May 28, 2013
30 Plex Human Luminex (Invitrogen Kit, Single Plate)
 30 Plex Human Luminex (Invitrogen Kit, Single Plate) 1. Defrost samples and bring to room temperature. 2. Bring Kit components to room temperature: Wash solution 20x. Assay Diluent. Incubation buffer.
30 Plex Human Luminex (Invitrogen Kit, Single Plate) 1. Defrost samples and bring to room temperature. 2. Bring Kit components to room temperature: Wash solution 20x. Assay Diluent. Incubation buffer.
Directions for running IC samples on the Shimadzu TOC analyzer. (5/18/2015 DER)
 Directions for running IC samples on the Shimadzu TOC analyzer. (5/18/2015 DER) Turn on the TOC analyzer by pressing the on switch located in the lower left corner of the panel on the front of the instrument.
Directions for running IC samples on the Shimadzu TOC analyzer. (5/18/2015 DER) Turn on the TOC analyzer by pressing the on switch located in the lower left corner of the panel on the front of the instrument.
Metzger Lab Protocol Book Written by Pierre Coutu 08/06/2003. Ionoptix Procedure
 1. Overview: Ionoptix Procedure The Ionoptix system allows the user to measure simultaneously unloaded sarcomere shortening and calcium fluorescence in isolated cardiac myocytes. The cells have to be plated
1. Overview: Ionoptix Procedure The Ionoptix system allows the user to measure simultaneously unloaded sarcomere shortening and calcium fluorescence in isolated cardiac myocytes. The cells have to be plated
Technical Bulletin. Guide for Using BD Cytometric Bead Array (CBA) Flex Sets with the BD Accuri C6 Flow Cytometer. Introduction
 Guide for Using BD Cytometric Bead Array (CBA) Flex Sets with the BD Accuri C6 Contents 1 Introduction 2 Materials and Methods 3 Setup Procedure Introduction BD Cytometric Bead Array (CBA) flex sets provide
Guide for Using BD Cytometric Bead Array (CBA) Flex Sets with the BD Accuri C6 Contents 1 Introduction 2 Materials and Methods 3 Setup Procedure Introduction BD Cytometric Bead Array (CBA) flex sets provide
Nikon Eclipse Ti2-E Widefield/Spinning Disk Confocal Microscope Standard Operation Protocol
 Nikon Eclipse Ti-E Widefield/Spinning Disk Confocal Microscope Standard Operation Protocol Please sign on the log sheet before switching on system. Turn on system Turn on A only if confocal mode or laser
Nikon Eclipse Ti-E Widefield/Spinning Disk Confocal Microscope Standard Operation Protocol Please sign on the log sheet before switching on system. Turn on system Turn on A only if confocal mode or laser
ab Firefly Cytometer Setup Particles
 Version 1 Last updated 12 October 2016 ab211043 Firefly Cytometer Setup Particles For cytometer performance optimization for use with Firefly Multiplex particles. This product is for research use only
Version 1 Last updated 12 October 2016 ab211043 Firefly Cytometer Setup Particles For cytometer performance optimization for use with Firefly Multiplex particles. This product is for research use only
Corrie Moreau Page 1 7/23/10
 Corrie Moreau Page 1 7/23/10 DNA Extractions using Qiagen DNeasy Kits with Extraction Beads Corrie Moreau Field Museum (September 2009) These are the instructions I use for DNA extractions of individual
Corrie Moreau Page 1 7/23/10 DNA Extractions using Qiagen DNeasy Kits with Extraction Beads Corrie Moreau Field Museum (September 2009) These are the instructions I use for DNA extractions of individual
V-GES operation. Technical Bulletin E-02 MATERIAL PROCEDURE
 V-GES operation MATERIAL BSA (1 mg/ml), 0.05 g BSA (Sigma-Aldrich Ltd., St Louis, MO; U.S.A.) dissolved in 50 ml ddh 2 O, aliquot 1 ml solution per Eppendorf and freeze in -20. Pre-stained marker (Bio-Rad,
V-GES operation MATERIAL BSA (1 mg/ml), 0.05 g BSA (Sigma-Aldrich Ltd., St Louis, MO; U.S.A.) dissolved in 50 ml ddh 2 O, aliquot 1 ml solution per Eppendorf and freeze in -20. Pre-stained marker (Bio-Rad,
LSM 780 Confocal Microscope Standard Operation Protocol
 LSM 780 Confocal Microscope Standard Operation Protocol Basic Operation Turning on the system 1. Sign on log sheet according to Actual start time 2. Check Compressed Air supply for the air table 3. Switch
LSM 780 Confocal Microscope Standard Operation Protocol Basic Operation Turning on the system 1. Sign on log sheet according to Actual start time 2. Check Compressed Air supply for the air table 3. Switch
LSM 800 Confocal Microscope Standard Operation Protocol
 LSM 800 Confocal Microscope Standard Operation Protocol Turning on the system 1. Switch on the Main switch (labeled 1 and 2 ) mounted on the wall. 2. Turn the Laser Key (labeled 3 ) 90 clockwise for power
LSM 800 Confocal Microscope Standard Operation Protocol Turning on the system 1. Switch on the Main switch (labeled 1 and 2 ) mounted on the wall. 2. Turn the Laser Key (labeled 3 ) 90 clockwise for power
AutoSeal FD 1506 Plus / FE 1506 Plus
 AutoSeal FD 1506 Plus / FE 1506 Plus FK / FL SERIES 06/2018 OPERATOR MANUAL FIRST EDITION TABLE OF CONTENTS DESCRIPTION 1 UNPACKING AND SET-UP 2 CONTROL PANEL 3 OPERATION 3 FOLD PLATE ADJUSTMENT 4 SETTING
AutoSeal FD 1506 Plus / FE 1506 Plus FK / FL SERIES 06/2018 OPERATOR MANUAL FIRST EDITION TABLE OF CONTENTS DESCRIPTION 1 UNPACKING AND SET-UP 2 CONTROL PANEL 3 OPERATION 3 FOLD PLATE ADJUSTMENT 4 SETTING
ChIP Protocol for fresh or frozen cross linked cells
 Prior to starting your ChIPs and Shearing Turn on sonifiers and cooling system allow system to reach -2 C before shearing Cool bench top centrifuge to 4 C Prepare all of your buffers with protease inhibitors
Prior to starting your ChIPs and Shearing Turn on sonifiers and cooling system allow system to reach -2 C before shearing Cool bench top centrifuge to 4 C Prepare all of your buffers with protease inhibitors
SOP-P051. Scanning of Optical Filters With USB2000. Objective: To determine the spectral transmittance properties of an optical filter.
 Purdue University Cytometry Laboratories SOP-P051 Scanning of Optical Filters With USB2000 Objective: To determine the spectral transmittance properties of an optical filter. Procedure: 1. Ensure that
Purdue University Cytometry Laboratories SOP-P051 Scanning of Optical Filters With USB2000 Objective: To determine the spectral transmittance properties of an optical filter. Procedure: 1. Ensure that
Trademarks Cytek, the Cytek logo, and all other trademarks are property of Cytek Biosciences Cytek
 Aurora User s Guide Copyrights 2017, Cytek Biosciences Inc. All rights reserved. No part of this publication may be reproduced, transmitted, transcribed, stored in retrieval systems, or translated into
Aurora User s Guide Copyrights 2017, Cytek Biosciences Inc. All rights reserved. No part of this publication may be reproduced, transmitted, transcribed, stored in retrieval systems, or translated into
RAITH e-line OPERATING INSTRUCTIONS
 RAITH e-line OPERATING INSTRUCTIONS 1) LOADING A SAMPLE a. Start the system i. On the Column PC (Right side monitor [R]), select the SmartSEM icon to on the desktop to begin the column software. ii. On
RAITH e-line OPERATING INSTRUCTIONS 1) LOADING A SAMPLE a. Start the system i. On the Column PC (Right side monitor [R]), select the SmartSEM icon to on the desktop to begin the column software. ii. On
Alon s SCN ChIP Protocol
 Prior to starting your ChIPs and Shearing 1. Turn on sonifiers and cooling system allow system to reach -1 C before shearing 2. Cool bench top centrifuge to 4 C 3. Prepare all of your buffers with protease
Prior to starting your ChIPs and Shearing 1. Turn on sonifiers and cooling system allow system to reach -1 C before shearing 2. Cool bench top centrifuge to 4 C 3. Prepare all of your buffers with protease
Olympus Time-lapse Microscope Basic operation
 Olympus Time-lapse Microscope Basic operation To start up the microscope 1. Switch on the Olympus UCB. (label as ) 1 Power Switch 2 2. Switch on the MT10. (label as ) Power Switch Page 1 of 18 3 3. Switch
Olympus Time-lapse Microscope Basic operation To start up the microscope 1. Switch on the Olympus UCB. (label as ) 1 Power Switch 2 2. Switch on the MT10. (label as ) Power Switch Page 1 of 18 3 3. Switch
Step 1: Set up the variables AB Design. Use the top cells to label the variables that will be displayed on the X and Y axes of the graph
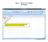 Step 1: Set up the variables AB Design Use the top cells to label the variables that will be displayed on the X and Y axes of the graph Step 1: Set up the variables X axis for AB Design Enter X axis label
Step 1: Set up the variables AB Design Use the top cells to label the variables that will be displayed on the X and Y axes of the graph Step 1: Set up the variables X axis for AB Design Enter X axis label
Procedure & Checklist - 2 kb Template Preparation and Sequencing
 Procedure & Checklist - 2 kb Template Preparation and Sequencing Before You Begin To perform this procedure, you must have the PacBio DNA Template Prep Kit (verify you have the correct kit for your insert
Procedure & Checklist - 2 kb Template Preparation and Sequencing Before You Begin To perform this procedure, you must have the PacBio DNA Template Prep Kit (verify you have the correct kit for your insert
BIOL110L-Cell Biology Lab Spring Quarter 2012 Module 3-4 Wednesday May 30, 2012
 BIOL110L-Cell Biology Lab Spring Quarter 2012 Module 3-4 Wednesday May 30, 2012 PART I: Isolation of over-expressed GFP-Karyopherins from yeast extracts by affinity capture on nucleoporins Summary: The
BIOL110L-Cell Biology Lab Spring Quarter 2012 Module 3-4 Wednesday May 30, 2012 PART I: Isolation of over-expressed GFP-Karyopherins from yeast extracts by affinity capture on nucleoporins Summary: The
BoTest Matrix E Botulinum Neurotoxin Detection Kit Protocol
 BoTest Matrix E Botulinum Neurotoxin Detection Kit Protocol 505 S. Rosa Road, Suite 105 Madison, WI 53719 1-608-441-8174 info@biosentinelpharma.com BioSentinel Part No: L1016, Release Date: May 29, 2014
BoTest Matrix E Botulinum Neurotoxin Detection Kit Protocol 505 S. Rosa Road, Suite 105 Madison, WI 53719 1-608-441-8174 info@biosentinelpharma.com BioSentinel Part No: L1016, Release Date: May 29, 2014
pluribead KIT Cell Separation Protocol M-Bead
 pluribead KIT Cell Separation Protocol M-Bead pluriselect@hiss-dx.de 15 14 13 12 11 9 8 7 6 5 4 3 2 1 50 40 30 20 2 Contents Contents pluribead Kit Components & Additional Materials 2 Separation Protocol
pluribead KIT Cell Separation Protocol M-Bead pluriselect@hiss-dx.de 15 14 13 12 11 9 8 7 6 5 4 3 2 1 50 40 30 20 2 Contents Contents pluribead Kit Components & Additional Materials 2 Separation Protocol
Introduction to QTO. Objectives of QTO. Getting Started. Requirements. Creating a Bill of Quantities. Updating an existing Bill of Quantities
 QTO User Manual Contents Introduction to QTO... 5 Objectives of QTO... 5 Getting Started... 5 QTO Manager... 6 QTO Layout... 7 Bill of Quantities... 8 Measure Folders... 9 Drawings... 10 Zooming and Scrolling...
QTO User Manual Contents Introduction to QTO... 5 Objectives of QTO... 5 Getting Started... 5 QTO Manager... 6 QTO Layout... 7 Bill of Quantities... 8 Measure Folders... 9 Drawings... 10 Zooming and Scrolling...
MAKE SURE YOUR SLIDES ARE CLEAN (TOP & BOTTOM) BEFORE LOADING DO NOT LOAD SLIDES DURING SOFTWARE INITIALIZATION
 Olympus VS120-L100 Slide Scanner Standard Operating Procedure Startup 1) Red power bar switch (behind monitor) 2) Computer 3) Login: UserVS120 account (no password) 4) Double click: WAIT FOR INITIALIZATION
Olympus VS120-L100 Slide Scanner Standard Operating Procedure Startup 1) Red power bar switch (behind monitor) 2) Computer 3) Login: UserVS120 account (no password) 4) Double click: WAIT FOR INITIALIZATION
BD OneFlow Setup Beads
 7/2014 23-15758-00 IVD BD OneFlow Setup Beads 25 tests per kit Catalog No. 658620 BD, BD Logo and all other trademarks are property of Becton, Dickinson and Company. 2014 BD Becton, Dickinson and Company
7/2014 23-15758-00 IVD BD OneFlow Setup Beads 25 tests per kit Catalog No. 658620 BD, BD Logo and all other trademarks are property of Becton, Dickinson and Company. 2014 BD Becton, Dickinson and Company
Setup Procedure for Beckman Coulter CytoFLEX Flow Cytometer
 Setup Procedure for Beckman Coulter CytoFLEX Flow Cytometer This part of the guide applies to Beckman Coulter flow cytometers using Cyto- Expert software version 1.2.8 and later. For CytoFLEX flow cytometers,
Setup Procedure for Beckman Coulter CytoFLEX Flow Cytometer This part of the guide applies to Beckman Coulter flow cytometers using Cyto- Expert software version 1.2.8 and later. For CytoFLEX flow cytometers,
Recording EPR spectra using the Loop Gap Resonator (LGR)
 Recording EPR spectra using the Loop Gap Resonator (LGR) This protocol gives step-by-step instructions for recording EPR spectra of spin labeled proteins (Nitroxide label like MTSSL) using the LGR assuming
Recording EPR spectra using the Loop Gap Resonator (LGR) This protocol gives step-by-step instructions for recording EPR spectra of spin labeled proteins (Nitroxide label like MTSSL) using the LGR assuming
DNA extraction Protocol for Agencourt Genfind v2 Blood and Serum Genomic DNA Isolation Kit
 DNA extraction Protocol for Agencourt Genfind v2 Blood and Serum Genomic DNA Isolation Kit Introduction The Agencourt Genfind v2 Blood & Serum DNA Isolation Kit utilizes Agencourt s patented SPRI paramagnetic
DNA extraction Protocol for Agencourt Genfind v2 Blood and Serum Genomic DNA Isolation Kit Introduction The Agencourt Genfind v2 Blood & Serum DNA Isolation Kit utilizes Agencourt s patented SPRI paramagnetic
COUNTING BACTERIA OBJECTIVES:
 COUNTING BACTERIA Many studies require the quantitative determination of bacterial populations. The two most widely used methods for determining bacterial numbers are the standard, or viable, plate count
COUNTING BACTERIA Many studies require the quantitative determination of bacterial populations. The two most widely used methods for determining bacterial numbers are the standard, or viable, plate count
illumina TruSeq RNA Sample Prep. (LT) Protocol 1
 illumina TruSeq RNA Sample Prep. (LT) Protocol 1 Performed using the TruSeq RNA Sample Preparation Kit (A cat#fc-122-1001, B cat#fc122-1002) Purify and Fragment mrna NOTE: Use 3ug of Total RNA to initiate
illumina TruSeq RNA Sample Prep. (LT) Protocol 1 Performed using the TruSeq RNA Sample Preparation Kit (A cat#fc-122-1001, B cat#fc122-1002) Purify and Fragment mrna NOTE: Use 3ug of Total RNA to initiate
CHM 152 Lab 1: Plotting with Excel updated: May 2011
 CHM 152 Lab 1: Plotting with Excel updated: May 2011 Introduction In this course, many of our labs will involve plotting data. While many students are nerds already quite proficient at using Excel to plot
CHM 152 Lab 1: Plotting with Excel updated: May 2011 Introduction In this course, many of our labs will involve plotting data. While many students are nerds already quite proficient at using Excel to plot
Ocean Optics R-2000 Raman Spectrometer Setup and Operating Instructions Arlen Viste and Deanna Donohoue April 2000 Update 2003, DEW
 Ocean Optics R-2000 Raman Spectrometer Setup and Operating Instructions Arlen Viste and Deanna Donohoue April 2000 Update 2003, DEW References Raman Systems R-2000 Operating Manual, Version 1.6, Ocean
Ocean Optics R-2000 Raman Spectrometer Setup and Operating Instructions Arlen Viste and Deanna Donohoue April 2000 Update 2003, DEW References Raman Systems R-2000 Operating Manual, Version 1.6, Ocean
Olympus xcellence Software - basic user guide
 Olympus xcellence Software - basic user guide This is a basic overview of setting up time lapse experiments using Olympus's xcellence software on BIU's IX81 inverted phase contrast system - the software
Olympus xcellence Software - basic user guide This is a basic overview of setting up time lapse experiments using Olympus's xcellence software on BIU's IX81 inverted phase contrast system - the software
Procedure & Checklist - 20 kb Template Preparation Using BluePippin Size-Selection System
 Procedure & Checklist - 20 kb Template Preparation Using BluePippin Size-Selection System Before You Begin To perform this procedure, you must have the PacBio DNA Template Prep Kit 2.0 (3 kb to 10 kb)
Procedure & Checklist - 20 kb Template Preparation Using BluePippin Size-Selection System Before You Begin To perform this procedure, you must have the PacBio DNA Template Prep Kit 2.0 (3 kb to 10 kb)
Using the zoom adjustment, zoom on the gel Adjust the tray on the VGAU 3000 to see the image of the gel in the viewfinder
 Operation of Vakili 3000 Gel Analysis Unit Both qualitative and quantitative analysis of electrophoresis experiments can be accomplished by using the Vakili 3000 Gel Analysis Unit. There are three steps
Operation of Vakili 3000 Gel Analysis Unit Both qualitative and quantitative analysis of electrophoresis experiments can be accomplished by using the Vakili 3000 Gel Analysis Unit. There are three steps
EKA Laboratory Muon Lifetime Experiment Instructions. October 2006
 EKA Laboratory Muon Lifetime Experiment Instructions October 2006 0 Lab setup and singles rate. When high-energy cosmic rays encounter the earth's atmosphere, they decay into a shower of elementary particles.
EKA Laboratory Muon Lifetime Experiment Instructions October 2006 0 Lab setup and singles rate. When high-energy cosmic rays encounter the earth's atmosphere, they decay into a shower of elementary particles.
The Revolve Feature and Assembly Modeling
 The Revolve Feature and Assembly Modeling PTC Clock Page 52 PTC Contents Introduction... 54 The Revolve Feature... 55 Creating a revolved feature...57 Creating face details... 58 Using Text... 61 Assembling
The Revolve Feature and Assembly Modeling PTC Clock Page 52 PTC Contents Introduction... 54 The Revolve Feature... 55 Creating a revolved feature...57 Creating face details... 58 Using Text... 61 Assembling
RENISHAW INVIA RAMAN SPECTROMETER
 STANDARD OPERATING PROCEDURE: RENISHAW INVIA RAMAN SPECTROMETER Purpose of this Instrument: The Renishaw invia Raman Spectrometer is an instrument used to analyze the Raman scattered light from samples
STANDARD OPERATING PROCEDURE: RENISHAW INVIA RAMAN SPECTROMETER Purpose of this Instrument: The Renishaw invia Raman Spectrometer is an instrument used to analyze the Raman scattered light from samples
Accutome Connect Visual Aid
 Accutome Connect Visual Aid Table of Contents A-Scan Mode Page 3 B-Scan Mode Page 15 UBM.. Page 23 B-Scan/UBM Recording.. Page 31 2 Accutome Connect: A-Scan 3 Probe Position 2. 1. Insert probe through
Accutome Connect Visual Aid Table of Contents A-Scan Mode Page 3 B-Scan Mode Page 15 UBM.. Page 23 B-Scan/UBM Recording.. Page 31 2 Accutome Connect: A-Scan 3 Probe Position 2. 1. Insert probe through
MC3 Motion Control System Shutter Stream Quickstart
 MC3 Motion Control System Shutter Stream Quickstart Revised 7/6/2016 Carousel USA 6370 N. Irwindale Rd. Irwindale, CA 91702 www.carousel-usa.com Proprietary Information Carousel USA has proprietary rights
MC3 Motion Control System Shutter Stream Quickstart Revised 7/6/2016 Carousel USA 6370 N. Irwindale Rd. Irwindale, CA 91702 www.carousel-usa.com Proprietary Information Carousel USA has proprietary rights
Leica SP8 TCS Users Manual
 Version : 07/08/0 Leica SP8 TCS Users Manual Start up:. Turn the PC Microscope, Scanner Power, Laser Power, and the Laser Emission key to on (bottom right of desk).. Turn on the fluorescent lamp (top left
Version : 07/08/0 Leica SP8 TCS Users Manual Start up:. Turn the PC Microscope, Scanner Power, Laser Power, and the Laser Emission key to on (bottom right of desk).. Turn on the fluorescent lamp (top left
MicroLab 500-series Getting Started
 MicroLab 500-series Getting Started 2 Contents CHAPTER 1: Getting Started Connecting the Hardware....6 Installing the USB driver......6 Installing the Software.....8 Starting a new Experiment...8 CHAPTER
MicroLab 500-series Getting Started 2 Contents CHAPTER 1: Getting Started Connecting the Hardware....6 Installing the USB driver......6 Installing the Software.....8 Starting a new Experiment...8 CHAPTER
Reference manual. EnVision. Software version 1.12
 2104-9020-02 March 2008 Reference manual EnVision Software version 1.12 PerkinElmer Life and Analytical Sciences, Wallac Oy, P.O. Box 10, FIN-20101 Turku, Finland. Tel: 358-2-2678111. Fax: 358-2-2678357
2104-9020-02 March 2008 Reference manual EnVision Software version 1.12 PerkinElmer Life and Analytical Sciences, Wallac Oy, P.O. Box 10, FIN-20101 Turku, Finland. Tel: 358-2-2678111. Fax: 358-2-2678357
Procedure & Checklist - 1 kb Template Preparation and Sequencing
 Procedure & Checklist - 1 kb Template Preparation and Sequencing Before You Begin To perform this procedure, you must have the PacBio Template Prep Kit. Fragment and Concentrate DNA Important: The distribution
Procedure & Checklist - 1 kb Template Preparation and Sequencing Before You Begin To perform this procedure, you must have the PacBio Template Prep Kit. Fragment and Concentrate DNA Important: The distribution
ScanArray Overview. Principle of Operation. Instrument Components
 ScanArray Overview The GSI Lumonics ScanArrayÒ Microarray Analysis System is a scanning laser confocal fluorescence microscope that is used to determine the fluorescence intensity of a two-dimensional
ScanArray Overview The GSI Lumonics ScanArrayÒ Microarray Analysis System is a scanning laser confocal fluorescence microscope that is used to determine the fluorescence intensity of a two-dimensional
Quick Guide. NucleoCounter NC-3000
 Quick Guide NucleoCounter NC-3000 Table of contents Setting up the FlexiCyte Protocol 2 Editing Image Capture and Analysis Parameters 3 Optimizing Exposure Time 4 Compensation for Spectral Overlap 6 Creating
Quick Guide NucleoCounter NC-3000 Table of contents Setting up the FlexiCyte Protocol 2 Editing Image Capture and Analysis Parameters 3 Optimizing Exposure Time 4 Compensation for Spectral Overlap 6 Creating
Conversational CAM Manual
 Legacy Woodworking Machinery CNC Turning & Milling Machines Conversational CAM Manual Legacy Woodworking Machinery 435 W. 1000 N. Springville, UT 84663 2 Content Conversational CAM Conversational CAM overview...
Legacy Woodworking Machinery CNC Turning & Milling Machines Conversational CAM Manual Legacy Woodworking Machinery 435 W. 1000 N. Springville, UT 84663 2 Content Conversational CAM Conversational CAM overview...
LSM 710 Confocal Microscope Standard Operation Protocol
 LSM 710 Confocal Microscope Standard Operation Protocol Basic Operation Turning on the system 1. Switch on Main power switch 2. Switch on System / PC power button 3. Switch on Components power button 4.
LSM 710 Confocal Microscope Standard Operation Protocol Basic Operation Turning on the system 1. Switch on Main power switch 2. Switch on System / PC power button 3. Switch on Components power button 4.
Procedure and Checklist - 20 kb Template Preparation Using BluePippin Size-Selection System
 Procedure and Checklist - 20 kb Template Preparation Using BluePippin Size-Selection System Before You Begin To perform this procedure, you must have the PacBio Template Prep Kit and have reviewed the
Procedure and Checklist - 20 kb Template Preparation Using BluePippin Size-Selection System Before You Begin To perform this procedure, you must have the PacBio Template Prep Kit and have reviewed the
Procedure & Checklist bp Amplicon Library Preparation and Sequencing
 Procedure & Checklist - 250 bp Amplicon Library Preparation and Sequencing Before You Begin To perform this procedure, you must have the PacBio Template Prep Kit. This procedure is optimized for SMRTbell
Procedure & Checklist - 250 bp Amplicon Library Preparation and Sequencing Before You Begin To perform this procedure, you must have the PacBio Template Prep Kit. This procedure is optimized for SMRTbell
Best Practices with the Bio-Plex system. Alasdair I Reid Ph.D. Field Application Specialist Bio-Rad Laboratories
 Best Practices with the Bio-Plex system Alasdair I Reid Ph.D. Field Application Specialist Bio-Rad Laboratories Outline of Content How to contact Bio-Rad Start up and Shutdown Procedures Maintenance, Calibration
Best Practices with the Bio-Plex system Alasdair I Reid Ph.D. Field Application Specialist Bio-Rad Laboratories Outline of Content How to contact Bio-Rad Start up and Shutdown Procedures Maintenance, Calibration
10 kb to 20 kb Template Preparation and Sequencing with Low-Input DNA
 Please note: the shared protocols described herein may not have been validated by Pacific Biosciences and are provided as-is and without any warranty. Use of these protocols is offered to those customers
Please note: the shared protocols described herein may not have been validated by Pacific Biosciences and are provided as-is and without any warranty. Use of these protocols is offered to those customers
Illumina TruSeq Stranded mrna (LT) Protocol 1
 Illumina TruSeq Stranded mrna (LT) Protocol 1 Performed using the TruSeq Stranded mrna Sample Preparation Kit (A cat#fc-122-2101, B cat#fc122-2102) Purify and Fragment mrna NOTE: Use 500ng of Total RNA
Illumina TruSeq Stranded mrna (LT) Protocol 1 Performed using the TruSeq Stranded mrna Sample Preparation Kit (A cat#fc-122-2101, B cat#fc122-2102) Purify and Fragment mrna NOTE: Use 500ng of Total RNA
Formaldehyde Cross-linking of Chromatin from Drosophila
 2 Formaldehyde Cross-linking of Chromatin from Drosophila Protocol from modencode IGSB University of Chicago originally written by Alex Crofts and Sasha Ostapenko and updated by Matt Kirkey. 1. Set centrifuge
2 Formaldehyde Cross-linking of Chromatin from Drosophila Protocol from modencode IGSB University of Chicago originally written by Alex Crofts and Sasha Ostapenko and updated by Matt Kirkey. 1. Set centrifuge
Creating a Color Compensation file for Roche LightCycler 480 vers.i & vers. II
 Creating a Color Compensation file for Roche LightCycler 480 vers.i & vers. II Due to ROX and Cy5 dye emmision spectra overlap Roche LightCycler 480 can pick up signals from a dye measured by another channel.
Creating a Color Compensation file for Roche LightCycler 480 vers.i & vers. II Due to ROX and Cy5 dye emmision spectra overlap Roche LightCycler 480 can pick up signals from a dye measured by another channel.
Assignment 5 due Monday, May 7
 due Monday, May 7 Simulations and the Law of Large Numbers Overview In both parts of the assignment, you will be calculating a theoretical probability for a certain procedure. In other words, this uses
due Monday, May 7 Simulations and the Law of Large Numbers Overview In both parts of the assignment, you will be calculating a theoretical probability for a certain procedure. In other words, this uses
OzE Field Modules. OzE Studio Series. OzE Studio OzE Studio Lite. Quick reference pages OzE Studio Data Entry (2pgs) OzE Preview.
 1 OzE Field Modules OzE Studio Series OzE Studio OzE Studio Lite Quick reference pages OzE Studio Data Entry (2pgs) OzE Preview OzE has been designed to accommodate many different fields of photography,
1 OzE Field Modules OzE Studio Series OzE Studio OzE Studio Lite Quick reference pages OzE Studio Data Entry (2pgs) OzE Preview OzE has been designed to accommodate many different fields of photography,
ISONIC PA AUT Spiral Scan Inspection of Tubular Parts Operating Manual and Inspection Procedure Rev 1.00 Sonotron NDT
 ISONIC PA AUT Spiral Scan Inspection of Tubular Parts Operating Manual and Inspection Procedure Rev 1.00 Sonotron NDT General ISONIC PA AUT Spiral Scan Inspection Application was designed on the platform
ISONIC PA AUT Spiral Scan Inspection of Tubular Parts Operating Manual and Inspection Procedure Rev 1.00 Sonotron NDT General ISONIC PA AUT Spiral Scan Inspection Application was designed on the platform
EDUCATION GIS CONFERENCE Geoprocessing with ArcGIS Pro. Rudy Prosser GISP CTT+ Instructor, Esri
 EDUCATION GIS CONFERENCE Geoprocessing with ArcGIS Pro Rudy Prosser GISP CTT+ Instructor, Esri Maintenance What is geoprocessing? Geoprocessing is - a framework and set of tools for processing geographic
EDUCATION GIS CONFERENCE Geoprocessing with ArcGIS Pro Rudy Prosser GISP CTT+ Instructor, Esri Maintenance What is geoprocessing? Geoprocessing is - a framework and set of tools for processing geographic
IDEXX-PACS * 4.0. Imaging Software. Quick Reference Guide
 4 IDEXX-PACS * 4.0 Imaging Software Quick Reference Guide Capturing Images Before you begin: Adjust the collimation properly. Make sure the body part you are imaging matches the exam type you have selected.
4 IDEXX-PACS * 4.0 Imaging Software Quick Reference Guide Capturing Images Before you begin: Adjust the collimation properly. Make sure the body part you are imaging matches the exam type you have selected.
EPSON Stylus Pro Quick Reference Guide
 EPSON Stylus Pro 10000 Quick Reference Guide Loading Roll Paper First you attach the paper roll to the spindle and place the spindle in the printer. Then you load the paper for printing. 4 Slide the movable
EPSON Stylus Pro 10000 Quick Reference Guide Loading Roll Paper First you attach the paper roll to the spindle and place the spindle in the printer. Then you load the paper for printing. 4 Slide the movable
FEI Quanta 200 ESEM Basic instructions
 FEI Quanta 200 ESEM Basic instructions Desktop and then start the UI. If the computer has restarted and you need to login, Username: supervisor and Password: supervisor Log-in to the Microscope using the
FEI Quanta 200 ESEM Basic instructions Desktop and then start the UI. If the computer has restarted and you need to login, Username: supervisor and Password: supervisor Log-in to the Microscope using the
INTRODUCTION TO DATA STUDIO
 1 INTRODUCTION TO DATA STUDIO PART I: FAMILIARIZATION OBJECTIVE To become familiar with the operation of the Passport/Xplorer digital instruments and the DataStudio software. INTRODUCTION We will use the
1 INTRODUCTION TO DATA STUDIO PART I: FAMILIARIZATION OBJECTIVE To become familiar with the operation of the Passport/Xplorer digital instruments and the DataStudio software. INTRODUCTION We will use the
When to Perform the Test CCD 4-Color and/or Seq Fill Capillary Run Modules
 To navigate within this document use the or keys ABI PRISM 310 Genetic Analyzer Test CCD 4-Color and Seq Fill Capillary Run Modules BEFORE PERFORMING ANY TROUBLESHOOTING WORK ON YOUR ABI PRISM 310 GENETIC
To navigate within this document use the or keys ABI PRISM 310 Genetic Analyzer Test CCD 4-Color and Seq Fill Capillary Run Modules BEFORE PERFORMING ANY TROUBLESHOOTING WORK ON YOUR ABI PRISM 310 GENETIC
Using Your Chip Priming Station
 Using Your Chip Priming Station The Chip Priming Station (5065-4401), is for use with the Agilent 2100 Bioanalyzer Analysis Kits. Refer to Figure 1 on page 2 for a picture of the Chip Priming Station.
Using Your Chip Priming Station The Chip Priming Station (5065-4401), is for use with the Agilent 2100 Bioanalyzer Analysis Kits. Refer to Figure 1 on page 2 for a picture of the Chip Priming Station.
Using Your Chip Priming Station
 s1 Using Your Chip Priming Station The Chip Priming Station, part number 5065-4401, is for use with the Agilent 2100 Bioanalyzer LabChip Kits. Refer to Figure 1 and 2 for a picture of the Chip Priming
s1 Using Your Chip Priming Station The Chip Priming Station, part number 5065-4401, is for use with the Agilent 2100 Bioanalyzer LabChip Kits. Refer to Figure 1 and 2 for a picture of the Chip Priming
AGENCOURT GENFIND Blood & Serum Genomic DNA Isolation Kit
 Blood & Serum Genomic DNA Isolation Kit Page 1 of 9 Please refer to http://www.agencourt.com/technical for updated protocols and refer to MSDS instructions when handling or shipping any chemical hazards.
Blood & Serum Genomic DNA Isolation Kit Page 1 of 9 Please refer to http://www.agencourt.com/technical for updated protocols and refer to MSDS instructions when handling or shipping any chemical hazards.
Image and Data Acquisition
 Image and Data Acquisition LCP Image Acquisition Procedures This section provides guidelines for scanning images that will be added to the LCP image archive. By scanning the image, we obtain a digital
Image and Data Acquisition LCP Image Acquisition Procedures This section provides guidelines for scanning images that will be added to the LCP image archive. By scanning the image, we obtain a digital
UV-Vis-NIR Spectrophotometer Quick Start Guide
 UV-Vis-NIR Spectrophotometer Quick Start Guide The following instructions are provided as a Quick Start Guide for powering up, running measurements, and shutting down the Lambda 950 UV-Vis Spectrophotometer.
UV-Vis-NIR Spectrophotometer Quick Start Guide The following instructions are provided as a Quick Start Guide for powering up, running measurements, and shutting down the Lambda 950 UV-Vis Spectrophotometer.
Procedure & Checklist - 10 kb Template Preparation and Sequencing (with Low-Input DNA)
 Procedure & Checklist - 10 kb Template Preparation and Sequencing (with Low-Input DNA) Before You Begin To perform this procedure, you must have the PacBio : Template Prep Kit DNA/Polymerase Binding Kit
Procedure & Checklist - 10 kb Template Preparation and Sequencing (with Low-Input DNA) Before You Begin To perform this procedure, you must have the PacBio : Template Prep Kit DNA/Polymerase Binding Kit
Atomic Force Microscopy (Bruker MultiMode Nanoscope IIIA)
 Atomic Force Microscopy (Bruker MultiMode Nanoscope IIIA) This operating procedure intends to provide guidance for general measurements with the AFM. For more advanced measurements or measurements with
Atomic Force Microscopy (Bruker MultiMode Nanoscope IIIA) This operating procedure intends to provide guidance for general measurements with the AFM. For more advanced measurements or measurements with
Installation Instructions: Epson R200 CFS
 Installation Instructions: Epson R200 CFS Photo Stylus R200 Installation Procedure Prerequisite - Before starting this installation, you MUST test your printer to make sure it is printing 100% correctly.
Installation Instructions: Epson R200 CFS Photo Stylus R200 Installation Procedure Prerequisite - Before starting this installation, you MUST test your printer to make sure it is printing 100% correctly.
Procedure & Checklist - 10 kb Template Preparation and Sequencing
 Procedure & Checklist - 10 kb Template Preparation and Sequencing Before You Begin To perform this procedure, you must have the PacBio Template Prep Kit. This procedure can be used to prepare 10 kb libraries
Procedure & Checklist - 10 kb Template Preparation and Sequencing Before You Begin To perform this procedure, you must have the PacBio Template Prep Kit. This procedure can be used to prepare 10 kb libraries
EPSON Stylus C64. Printer Parts. Printer Specifications. Accessories. Media. Printing. Ink Cartridges
 Printer Parts Left edge guide support Printer cover Output tray Ink cartridges Output tray extension Media EPSON paper name Size Part number Premium Bright White Letter S041586 Photo Quality Ink Jet Letter
Printer Parts Left edge guide support Printer cover Output tray Ink cartridges Output tray extension Media EPSON paper name Size Part number Premium Bright White Letter S041586 Photo Quality Ink Jet Letter
IENG 475 Computer-Controlled Manufacturing Systems 2/7/2017. Lab 03: Manual Milling and Turning Operations
 I. Purpose Lab 03: Manual Milling and Turning Operations A.) B.) C.) D.) Provide an overview of safety considerations for the CNC Mill Provide manual experience using the laboratory s CNC Mill Provide
I. Purpose Lab 03: Manual Milling and Turning Operations A.) B.) C.) D.) Provide an overview of safety considerations for the CNC Mill Provide manual experience using the laboratory s CNC Mill Provide
Subdivision Cross Sections and Quantities
 NOTES Module 11 Subdivision Cross Sections and Quantities Quantity calculation and cross section generation are required elements of subdivision design projects. After the design is completed and approved
NOTES Module 11 Subdivision Cross Sections and Quantities Quantity calculation and cross section generation are required elements of subdivision design projects. After the design is completed and approved
BIO 365L Neurobiology Laboratory. Training Exercise 1: Introduction to the Computer Software: DataPro
 BIO 365L Neurobiology Laboratory Training Exercise 1: Introduction to the Computer Software: DataPro 1. Don t Panic. When you run DataPro, you will see a large number of windows, buttons, and boxes. In
BIO 365L Neurobiology Laboratory Training Exercise 1: Introduction to the Computer Software: DataPro 1. Don t Panic. When you run DataPro, you will see a large number of windows, buttons, and boxes. In
PARP1-Trap_A for Immunoprecipitation of PARP1- Fusion Proteins from cell extract
 PARP1-Trap_A for Immunoprecipitation of PARP1- Fusion Proteins from cell extract Only for research applications, not for diagnostic or therapeutic use. Introduction Specificity Poly(ADP-ribose) polymerase
PARP1-Trap_A for Immunoprecipitation of PARP1- Fusion Proteins from cell extract Only for research applications, not for diagnostic or therapeutic use. Introduction Specificity Poly(ADP-ribose) polymerase
Operating Checklist for using the Scanning Electron Microscope, JEOL JSM 6400.
 Smith College August 2005 Operating Checklist for using the Scanning Electron Microscope, JEOL JSM 6400. CONTENT, page no. Pre-Check, 1 Specimen Insertion, 1 Startup, 2 Filament Saturation, 2 Beam Alignment,
Smith College August 2005 Operating Checklist for using the Scanning Electron Microscope, JEOL JSM 6400. CONTENT, page no. Pre-Check, 1 Specimen Insertion, 1 Startup, 2 Filament Saturation, 2 Beam Alignment,
Series. Photo Printer. Direct Printing Guide
 Series Photo Printer Direct Printing Guide Contents Operation Panel and Menu Display Contents Operation Panel Names and Functions..................................................2 Menu Displays......................................................................4
Series Photo Printer Direct Printing Guide Contents Operation Panel and Menu Display Contents Operation Panel Names and Functions..................................................2 Menu Displays......................................................................4
Physics 253 Fundamental Physics Mechanic, September 9, Lab #2 Plotting with Excel: The Air Slide
 1 NORTHERN ILLINOIS UNIVERSITY PHYSICS DEPARTMENT Physics 253 Fundamental Physics Mechanic, September 9, 2010 Lab #2 Plotting with Excel: The Air Slide Lab Write-up Due: Thurs., September 16, 2010 Place
1 NORTHERN ILLINOIS UNIVERSITY PHYSICS DEPARTMENT Physics 253 Fundamental Physics Mechanic, September 9, 2010 Lab #2 Plotting with Excel: The Air Slide Lab Write-up Due: Thurs., September 16, 2010 Place
General Help. Last revised: Winter When I try to print something on the computer, it appears to work, but nothing comes out of the printer.
 General Help Last revised: Winter 2015 Problem Solution When I try to print something on the computer, it appears to work, but nothing comes out of the printer. See the next item. When I try to print something
General Help Last revised: Winter 2015 Problem Solution When I try to print something on the computer, it appears to work, but nothing comes out of the printer. See the next item. When I try to print something
Procedure & Checklist >20 kb Template Preparation Using BluePippin Size-Selection System (15-20 kb Cutoff) for Sequel Systems
 Procedure & Checklist >20 kb Template Preparation Using BluePippin Size-Selection System (15-20 kb Cutoff) for Sequel Systems Before You Begin To perform this procedure, you must have the PacBio Template
Procedure & Checklist >20 kb Template Preparation Using BluePippin Size-Selection System (15-20 kb Cutoff) for Sequel Systems Before You Begin To perform this procedure, you must have the PacBio Template
Microwell-Seq. High-throughput Single Cell RNA-Seq Kit. Protocol. Index
 Microwell-Seq High-throughput Single Cell RNA-Seq Kit Protocol Index 1 Introduction 2 Kit Reagent 3 Store 4 Application 5 Prepared materials 6 Note 7 Preparation 8 Workflow 1 1 Introduction Microwell-Seq
Microwell-Seq High-throughput Single Cell RNA-Seq Kit Protocol Index 1 Introduction 2 Kit Reagent 3 Store 4 Application 5 Prepared materials 6 Note 7 Preparation 8 Workflow 1 1 Introduction Microwell-Seq
Using the Hitachi 3400-N VP-SEM
 Using the Hitachi 3400-N VP-SEM Opening the Chamber to Load Specimens (This may also be done later using the software) 1. Click the AIR button on the front of the machine: 2. Wait a few minutes until you
Using the Hitachi 3400-N VP-SEM Opening the Chamber to Load Specimens (This may also be done later using the software) 1. Click the AIR button on the front of the machine: 2. Wait a few minutes until you
Scratch Assay Device for Wound Healing Studies
 Scratch Assay Device for Wound Healing Studies By Team Cell Scratcher: Hannah Morgan, Charles Merchant, and Zachariah Cribbin 1 Quick Start Guide 2 Comments: _ Table of Contents Cover Page 1 1.0 Quick
Scratch Assay Device for Wound Healing Studies By Team Cell Scratcher: Hannah Morgan, Charles Merchant, and Zachariah Cribbin 1 Quick Start Guide 2 Comments: _ Table of Contents Cover Page 1 1.0 Quick
Type of minimal media was selected based on the strain of E. coli being used: 5alpha M9 glycerol + Thiamine 1mM Thiamine (light-sensitive)
 Small-molecule Induction Colonies for induction were selected based on colony PCR results. 5uL of diluted colony (1 colony in 10uL NFW) was inoculated and incubated at 37 C / 250rpm overnight in 3.5mL
Small-molecule Induction Colonies for induction were selected based on colony PCR results. 5uL of diluted colony (1 colony in 10uL NFW) was inoculated and incubated at 37 C / 250rpm overnight in 3.5mL
Timekeeper/Statistical tool for Basketball Sponsor: Prof. Wayne Dyksen & MSU Basketball Team Spring User Guide
 Timekeeper/Statistical tool for Basketball Sponsor: Prof. Wayne Dyksen & MSU Basketball Team Spring 2004 User Guide Team 2 Edward Bangs Bryan Berry Chris Damour Kim Monteith Jonathan Szostak 1 Table of
Timekeeper/Statistical tool for Basketball Sponsor: Prof. Wayne Dyksen & MSU Basketball Team Spring 2004 User Guide Team 2 Edward Bangs Bryan Berry Chris Damour Kim Monteith Jonathan Szostak 1 Table of
Procedure & Checklist - Preparing SMRTbell Libraries using PacBio Barcoded Adapters for Multiplex SMRT Sequencing
 Procedure & Checklist - Preparing SMRTbell Libraries using PacBio Barcoded Adapters for Multiplex SMRT Sequencing Before You Begin This document describes methods for generating SMRTbell libraries using
Procedure & Checklist - Preparing SMRTbell Libraries using PacBio Barcoded Adapters for Multiplex SMRT Sequencing Before You Begin This document describes methods for generating SMRTbell libraries using
Excel Lab 2: Plots of Data Sets
 Excel Lab 2: Plots of Data Sets Excel makes it very easy for the scientist to visualize a data set. In this assignment, we learn how to produce various plots of data sets. Open a new Excel workbook, and
Excel Lab 2: Plots of Data Sets Excel makes it very easy for the scientist to visualize a data set. In this assignment, we learn how to produce various plots of data sets. Open a new Excel workbook, and
CRL MASS SPECTROMETRY FACILITY INSTRUMENT USER MANUAL MALDI MICRO. Operating Instructions. Basement Spec Lab:
 CRL MASS SPECTROMETRY FACILITY INSTRUMENT USER MANUAL MALDI MICRO Operating Instructions Basement Spec Lab: 00.097 crs Page of 2 This is a guide to using the MALDI Micro for those who have received training.
CRL MASS SPECTROMETRY FACILITY INSTRUMENT USER MANUAL MALDI MICRO Operating Instructions Basement Spec Lab: 00.097 crs Page of 2 This is a guide to using the MALDI Micro for those who have received training.
Standard Operating Procedure
 RIT MULTIDISCIPLINARY SENIOR DESIGN 2010 Standard Operating Procedure Baja Water Propulsion Test Stand This SOP specifies how to assemble, use, troubleshoot, and disassemble the water propulsion system
RIT MULTIDISCIPLINARY SENIOR DESIGN 2010 Standard Operating Procedure Baja Water Propulsion Test Stand This SOP specifies how to assemble, use, troubleshoot, and disassemble the water propulsion system
Procedure & Checklist bp Template Preparation and Sequencing
 Procedure & Checklist - 500 bp Template Preparation and Sequencing Before You Begin To perform this procedure, you must have the PacBio Template Prep Kit. This procedure is optimized for SMRTbell template
Procedure & Checklist - 500 bp Template Preparation and Sequencing Before You Begin To perform this procedure, you must have the PacBio Template Prep Kit. This procedure is optimized for SMRTbell template
ClonePix TM FL Software Applications Manual
 ClonePix TM FL Software Applications Manual Software Release: 1.2.13.971 07MAN1053.A2 Effective Date: 01-Jul-10 Contents Overview...5 Workflows...6 Pick Run...6 Batch Pick Run...7 Imaging Run...8 Start
ClonePix TM FL Software Applications Manual Software Release: 1.2.13.971 07MAN1053.A2 Effective Date: 01-Jul-10 Contents Overview...5 Workflows...6 Pick Run...6 Batch Pick Run...7 Imaging Run...8 Start
NCRC Animal Imaging Procedures
 1.0 Purpose 2.0 Scope The purpose of SOP 6.8 is to describe procedures for imaging on small animals in NCRC. SOP 6.8 is intended to cover all resources, personnel and equipment in the BCR laboratory and
1.0 Purpose 2.0 Scope The purpose of SOP 6.8 is to describe procedures for imaging on small animals in NCRC. SOP 6.8 is intended to cover all resources, personnel and equipment in the BCR laboratory and
AxioVision 4.5 Brightfield Image Capture Procedure
 AxioVision 4.5 Brightfield Image Capture Procedure 1. STARTING-UP PROCEDURE: Remove blue dust cover and place on shelf under microscope. Turn on the halogen lamp by pushing the switch at the back right
AxioVision 4.5 Brightfield Image Capture Procedure 1. STARTING-UP PROCEDURE: Remove blue dust cover and place on shelf under microscope. Turn on the halogen lamp by pushing the switch at the back right
Star Defender. Section 1
 Star Defender Section 1 For the first full Construct 2 game, you're going to create a space shooter game called Star Defender. In this game, you'll create a space ship that will be able to destroy the
Star Defender Section 1 For the first full Construct 2 game, you're going to create a space shooter game called Star Defender. In this game, you'll create a space ship that will be able to destroy the
CREATING (AB) SINGLE- SUBJECT DESIGN GRAPHS IN MICROSOFT EXCEL Lets try to graph this data
 CREATING (AB) SINGLE- SUBJECT DESIGN GRAPHS IN MICROSOFT EXCEL 2003 Lets try to graph this data Date Baseline Data Date NCR (intervention) 11/10 11/11 11/12 11/13 2 3 3 1 11/15 11/16 11/17 11/18 3 3 2
CREATING (AB) SINGLE- SUBJECT DESIGN GRAPHS IN MICROSOFT EXCEL 2003 Lets try to graph this data Date Baseline Data Date NCR (intervention) 11/10 11/11 11/12 11/13 2 3 3 1 11/15 11/16 11/17 11/18 3 3 2
Getting Started Guide
 SOLIDWORKS Getting Started Guide SOLIDWORKS Electrical FIRST Robotics Edition Alexander Ouellet 1/2/2015 Table of Contents INTRODUCTION... 1 What is SOLIDWORKS Electrical?... Error! Bookmark not defined.
SOLIDWORKS Getting Started Guide SOLIDWORKS Electrical FIRST Robotics Edition Alexander Ouellet 1/2/2015 Table of Contents INTRODUCTION... 1 What is SOLIDWORKS Electrical?... Error! Bookmark not defined.
RISK OF SHOCK: DO NOT WIPE DOWN ANY ELECTRICAL COMPONENTS. ALWAYS KEEP AWAY FROM ALL AREAS WHERE ELECTRONIC COMPONENTS ARE INSTALLED.
 Maintenance General Cleaning Waste material from the printing process can accumulate inside the printer. Using a slightly damp, lint-free cloth, wipe the interior of the CubePro including the print plate,
Maintenance General Cleaning Waste material from the printing process can accumulate inside the printer. Using a slightly damp, lint-free cloth, wipe the interior of the CubePro including the print plate,
