United States Patent (19)
|
|
|
- Kerry Norton
- 5 years ago
- Views:
Transcription
1 United States Patent (19) Story et al. 11 Patent Number: () Date of Patent: k Oct. 2, 1984 (54) 75 (73) * (51) SOAP MAKING PROCESS Inventors: Julian R. Story, Fountain Hills; E. Gary Myers, Scottsdale, both of Ariz. Assignee: Notice: Appl. No.: 396,543 Armour-Dial, Inc., Phoenix, Ariz. The portion of the term of this patent subsequent to Aug. 9, 00 has been disclaimed. Filed: Jul. 14, 1982 Related U.S. Application Data Continuation-in-part of Ser. No. 291,525, Aug. 10, 1981, Pat. No. 4,397,760, Int. C.... C11D 13/10 U.S. C /369; 252/108; 252/370; 260/415; 260/416; 260/417; 260/418; 366/222; 366/224; 366/105; 366/12; 366/13; 366/298 Field of Search /369, 370, 108; 260/415, 416, 417, 418; 366/222, 224, 105, 12, 13, ) References Cited U.S. PATENT DOCUMENTS 1,722,687 7/1929 Spensley /369 2,730,539 1/1956 Bradford /47 2,753,363 7/1956 Winer /43 3,657,146 4/1972 Fransen et al /369 3,674,241 7/1972 Eirich et al /105 4,294,771 10/1981 Pietralia et al /43 Primary Examiner-John E. Kittle Assistant Examiner-Robert A. Wax Attorney, Agent, or Firm-Frank T. Barber; Richard G. Harrer 57 ABSTRACT A process for the preparation of soap and soap/syn thetic detergent products from raw materials normally employed in the manufacture of such products includ ing fatty acids, triglycerides and caustic or alkali by subjecting such raw materials to intensive countercur rent mixing whereby saponification takes place in a relatively short time to yield a product, preferably in granular or powder form, which requires no further drying for most uses. The resulting product can, if de sired, be then subjected to plodding, extrusion and stamping to form soap in bar form. The starting material can also be a mixture of such raw materials where neu tralization has proceeded to some degree, preferably the neat soap stage. 7 Claims, 12 Drawing Figures
2 U.S. Patent Oct. 2, 1984 Sheet 1 of 3 N
3 U.S. Patent Oct. 2, 1984 Sheet 2 of 3
4 U.S. Patent Oct. 2, 1984 Sheet 3 of 3
5 1 SOAP MAKNG PROCESS BACKGROUND This is a continuation-in-part of application Ser. No. 291,525 filed Aug. 10, 1981, now U.S. Pat. No. 4,397,760. This invention relates to energy saving, rapid pro cesses for the preparation of soap and soap/synthetic detergent products. More particularly, the invention relates to the use of countercurrent mixing to produce soap, usually in granular form, using as starting materi als the raw materials normally employed in making soap or from a mixture of such materials where neutraliza tion is essentially completed, that is, the so-called neat soap stage. Although soap can be made by a number of different techniques, today its commercial manufacture basically involves either some type of batch (kettle) saponifica tion or a continuous process which includes the splitting of fats into fatty acids and glycerine and then the neu tralization of such fatty acids with caustic (usually ei ther sodium hydroxide or potassium hydroxide) con taining the proper amount of water to yield a neat soap containing about 30 percent by weight of moisture. While the most modern way to make soap is neutraliz ing fatty acids, considerable soap is still made by batch techniques which involve "cold process saponifica tion', "semi-boiled saponification' and a so-called 'ket tle process'. The cold process saponification is the sim plest of the batch procedures and since neither lyes nor nigre are separated, the glycerol and impurities from the fats remain in the soap. The charge of fat is simply melted in a vessel equipped with a mechanical stirrer and the calculated amounts of caustic soda solution is added with vigorous stirring. The fats and oils are mixed for a short time, usually from about ten minutes to one hour, or before the mix becomes too viscous to pour. At this time the saponification is about 90% com plete. The mix is then poured into a frame and stored about two days to a week until hard. During this aging period, the saponification is completed. The semi-boiled saponification technique is similar to the cold process, although a higher temperature is used to speed saponifi cation and permit adjustment of the alkali content be fore framing. The fat charge and alkali (which may be caustic potash when soft soaps are desired) are thor oughly mixed at ' F. until the soap becomes smooth. No glycerine is recovered in this process. The kettle process usually involves recovery of the glycer ine. In this process fat and a relatively weak solution of sodium hydroxide are pumped into the kettle simulta neously. As soon as the dilute caustic mixes with the fat, saponification starts. The liquid mass is boiled by the admission of steam at the bottom of the kettle and as saponification proceeds, stronger caustic is added grad ually until the saponification is almost complete. The soap is then "salted out' or "grained out' by the addi tion of a large amount of salt; the sodium soap, being insoluble in the concentrated salt solution and of a lower density rises to the top of the kettle and the salt solution containing salt, glycerine, impurities and excess alkali collect at the bottom of the vessel. The bottom brine layer is drawn off and then water and an excess of... lye are added to the soap remaining in the kettle. The mixture is boiled with steam to saponify the last traces of fat. The solution which collects at the bottom of the kettle during the subsequent settling process is drawn off. Brine is then added to the soap; the mass is boiled and allowed to stand until brine washed soap rises to the top of the kettle. The brine washing is repeated several times with fresh brine until the excess alkali and glycer 50 ine in soap are reduced to a minimum. The neat soap is then sent to dryers such as a Proctor-Swartz. No matter what soap making procedure is employed, the end product is neat soap which is usually subjected to further processing. For example, when neat soap is to be further processed to form bars or flakes, the water content of the neat soap, which is usually about 30% by weight, must be reduced to the range of about 10- percent by weight. This drying can be accomplished in a number of ways. In one procedure the neat soap flows onto a so-called chill roll which spreads the fluid soap into a thin film which then solidifies. The solidified soap film is removed in ribbon form and then oven dried to the required moisture content. More modern techniques utilize vacuum spray dryers to reduce the moisture content to a proper level. Following drying, the soap is passed to an amalgamator where perfume, color and other soap addivites are mixed into the soap mass and from there the soap can then be milled and plodded. The final steps to bar soap manufacture include the extrusion of the plodded soap through a tapered outlet to form a continuous log followed by cutting, stamping and finally packaging. It will be appreciated that in conventional soap bar/soap flake manufacturing prac 65 tices, the drying and plodding steps are energy-inten sive and very time consuming and it would be ex tremely desirable to develop a soap-making process which would either substantially reduce or eliminate the drying operation which is currently required to produce most soap products. Various proposals have been advanced to solve the problem of producing low moisture soap by eliminating or substantially reducing the energy required for drying but so far as we know none have really proved to be commercially feasible. For example, U.S. Pat. No. 2,730,539 discloses a method of saponifying fat such as tallow or vegetable oils with caustic to form a low moisture content soap using a "muller' type mixer. The soap making ingredient, such as tallow, and a solution of caustic soda are introduced into the muller mixer and subjected to a shearing and smearing action by the mull ing action of the heavy wheels rolling over the materials in the pan. According to the patent, a high order of mechanical pressure is applied to the soap-forming in gredients which results in a soap which is said to be suitable for plodding and then stamping into bars. Also, U.S. Pat. No. 3,657,146 discloses a process for the direct production of soap from fatty acids and caus tic in a pressure vessel at about 2 to 10 atmospheres and at a temperature ranging from 1 to 180 C. The pro cess is said to produce a soap having not more than about 25 percent water content and in this connection example 2 of said patent shows a soap which contains 9 percent of free fatty acids and 15 percent of water. In addition, the use of a two-stage saponification procedure is disclosed in U.S. Pat. No. 2,753,363. The initial reaction takes place between the fatty acids and a dry, alkalimetal carbonate such as sodium carbonate to achieve a partial saponification. Following this the par tially saponified mass is treated with aqueous alkali metal hydroxide to complete the reaction. U.S. Pat. No. 1,722,687 discloses the use of a high speed centrifugal pinned disc mill to make framed soaps,
6 3 soft soaps and dry soap powders. In the process the soap making ingredients are introduced into the mill and the lower rotating disc is run at very high speed causing a beating action of the reactants by the lower rotating pins and upper stationary pins. OBJECTS OF THE INVENTION It is an object of the present invention to provide a process for the production of low moisture water solu ble soaps from raw materials normally employed in soap manufacture including triglycerides, fatty acids and caustic and or alkalis such as triethanolamine which substantially reduces or eliminates entirely the need to dry the soap by conventional means prior to forming it into bars and the like. A further object is to provide a rapid process for making low moisture soap under ambient pressure and temperature conditions where stoichiometric amount of triglycerides or fatty acid and caustic can be processed to produce non-tacky soap in granular form having a typical moisture content of about percent or less. Another object is to provide a rapid process for the production of low moisture soap in the form of granules which eliminates the need to process the soap through an amalgamator and one of the plodding steps. A still further object is to provide a process for the production of a soap/synthetic detergent product which eliminates the need to dry the product by con ventional means prior to forming it into bars and the like. Still another object is to provide a process for the production of low moisture soap in the form of granules or powder from a mixture of conventional soap making materials such as fatty acids, triglycerides, caustic and the like where neutralization of the fatty acids and/or triglycerides is essentially completed, that is, the neat Soap stage. Other objects of this invention will become apparent as the specification proceeds. SUMMARY OF THE INVENTION We have discovered that high-quality soap can be produced by combining an appropriate source of long chain monocarboxylic acids such as triglycerides. or fatty acids and caustic in a mixing chamber so that the triglycerides and/or fatty acids and caustic are sub jected to an intensive countercurrent mixing whereby the triglycerides and/or fatty acids and caustic are sa ponified in a short period of time to yield a low moisture soap, preferably in granule form, which requires no further drying for most uses. As used herein the expres sion "fatty acid source' means the raw materials which are customarily employed in soap manufacture such as the naturally occurring fats and oils which are triglycer ides with three fatty groups randomly esterified with glycerol (tallow, lard, coconut oil, palm kernal oils and the like) or the fatty acids which result from the split ting or hydrolysis of the triglyceride fats and oils or the fatty acids derived from synthetic sources. The expression "saponify or "saponification' means either the neutralization of fatty acids to produce soap or the saponification of fats and/or oils to produce soap. By intensive countercurrent mixing we mean causing a liquid stream of the reactants to rapidly move in a circu lar direction (e.g. clockwise) within a mixing vessel and at the same time bringing this rapidly moving stream into contact with mixing means rotating rapidly in a direction counter (e.g. counterclockwise) to the flow of the reactant stream. This head on meeting of the rap idly, circular moving stream with the counter rotating mixing means creates a generally rotary movement of the reactants within the vessel appearing much like an eddy stream or whirlpool. The reaction time can be shortened by mounting the counter-rotating means ec centrically within the vessel and at a distance from the wall of the vessel. When the counter-rotating means are so mounted it is desirable to provide deflecting means within the vessel which serve to direct the stream of reactants to the counter-rotating means. The reactants are thereby directed into several counter moving paths and brought together again at high impact velocity. We have also discovered that intensive counter-cur rent mixing can be employed to produce soap in granu lar or powder form from a saponifiable mixture of a fatty acid source and caustic where saponification of said mixture has proceeded to some degree, preferably to the neat soap stage. By neat soap we mean the prod uct resulting from the reaction of a fatty acid source with a suitable caustic or alkali and where neutralization is essentially completed, the product usually containing about 30% by weight of water. Thus the neat soap prepared by various prior processes such as: the contin uous process which includes splitting fats into fatty acids and glycerine and then neutralization of the acids with caustic, the cold process saponification, the semi boiled saponification process or the kettle process can be subjected to intensive counter current mixing to yield a low moisture soap in granule or even powder form. GENERAL DESCRIPTION OF THE DRAWINGS FIG. 1 is a perspective view of mixing equipment adapted to provide the intensive countercurrent mixing of the fatty acid sources and caustic. FIG. 2 is a horizontal sectional view taken substan tially on the line 2-2 of FIG.1. FIG.3 is a fragmentary sectional view taken substan tially on the line 3-3 of FIG. 2. FIG. 4 and FIG. 5 are perspective views of rotors which can be employed in the mixing equipment shown in FIG. 1 and FIG. 2. FIG. 6 is a perspective view of a portable mixer which can provide countercurrent mixing on highly. reduced scale. FIG. 7 and FIG. 12 are perspective views of rotors useful in the mixing equipment shown in FIG. 11. FIG. 8 is a sectional view taken substantially on the line 8-8 of FIG. 7. FIG.9 is a perspective view of a mixing tool which is mounted within the mixing equipment shown in FIG. 11. FIG. 10 is an enlarged view of the mixing plow shown in FIG. 9. Referring to the drawings, FIG. 1 shows an embodi ment of the mixing equipment useful in our invention and is designated generally at 10. The mixer 10 can be described as a mixing pan 11 rotatably mounted on frame 12 and surrounded by metal shroud 13. Access to the interior of the mixer and more specifically to the mixing pan is provided by hinged loading door 14. At the top of the shroud 13 are ports 15 and 16 which can be used to introduce materials and/or air directly to the mixing pan or to serve as an exit for gases which may develop during the saponification process. The equip ment is further provided with a water tight discharge gate 29 at the bottom of the mixing pan and this dis
7 5 charge gate is controlled by handle 17. The discharge gate allows for removal of the soap after saponification has been completed. Although not shown, the mixing pan 11 is belt driven by a separate motor mounted adjacent to the mixing pan. The required horsepower of this motor is of course dependent on the size of the mixing pan employed and the characteristics of the batch of ingredients being processed. As previously mentioned, the mixing pan is rotatably mounted and in the particular embodiment 10 illustrated in FIG. 2, rotates in a clockwise manner. Mounted to the top of mixer 10 and eccentrically within mixing pan 11 is rotor assembly 18. Although not shown, this assembly is normally provided with a sepa rate variable speed motor so that the speed of the rotor 15 assembly may be changed as desired. The rotor assem bly consists of attachment member 19 for securing the assembly to the drive motor and shaft. Various types of mixing tools may be mounted on shaft and FIG. 4 and FIG. 5 show two examples. The mixing tool of FIG. 4 consists of generally circular plate 21 to which are mounted pins 22. Weights 23 can be used to counter balance the rotor assembly if this is required. In FIG. 5, the mixing tool consists of two pairs of arms or knives 25 which are mounted at substantially right angles to 25 each other and can be provided with balance weights 26 to counterbalance the assembly if such is necessary. FIG.2 and FIG. 3 show a rotor assembly as described in FIG. 4 and it will be noted that the assembly is eccen trically mounted within the mixing pan and rotates in a direction counter to the direction of rotation of the mixing pan. Mounted within mixing pan 11 are means to insure that the materials within the mixing pan are subjected to the intensive countercurrent mixing operation. These means are secured to the top part of the mixing equip ment immediately above the mixing pan and, as shown in FIG. 2 and FIG. 3 consist of a pan wall wiper 27 and pan bottom deflector 28 which is attached to the pan wall wiper. As the pan rotates in a clockwise direction, the pan wall wiper scrapes the reactant materials from the pan wall and directs such materials to the rotor area for improved mixing. In the same fashion, bottom de flector 28 gathers the reactant materials and directs them to the area of intensive mixing ensuring that all materials are subjected to the mixing process. The mixing equipment depicted in FIG. 11 operates on the same principles as that of FIG. 1 but is designed to process much larger quantities of fatty acid sources and caustic or neat soap. Whereas the equipment shown in FIGS. 1-3 will handle up to about 100 pounds per batch, the equipment of FIG. 11 will handle about 800 pounds. The mixer shown generally at 30 has a rotat ably mounted mixing pan 31 mounted on frame 32. Access to the interior of the mixer is provided by hinged door 34. At the top are ports and 36 which are used to introduce air under pressure to the mixing chamber 37 and, in the case of port 36 to serve as an exit for the pressurized air. Although not shown the mixer is provided with a watertight discharge gate at the bottom of the mixing pan to remove the soap granules. Within the interior of the mixer is an eccentrically mounted rotating mixing tool 38 which is shown in greater detail in FIG. 9 and FIG. 10. Mixing tool 38 is provided with : a kneading bar 39 and mixing plow 40 and is powered by motor 40a. As shown most clearly in FIG. 9 the mixing tool 38 rotates in a direction 41a which is counter to the direction of rotation of pan which is shown by arrow 41. Thus as shown the pan rotates in a clockwise direction, mixing tool 38 rotates in a counter clockwise direction. Also mounted within the mixer 30 is a high speed rotor assembly 42. This rotor is also designed to rapidly rotate in a direction counter to that of the pan. Various types of rotors may be used and FIG. 7 and FIG. 12 are two examples. The rotor of FIG. 7 consists of attachment member 43 for securing the rotor assembly to the upper portion of mixer 30, a drive motor (not shown) and shaft 44. Mounted at the end of shaft 44 is a circular plate to which are mounted a series of pins 46 and 47. Pins 46 are some what shorter than pins 47. Pins 47 are also provided with a generally rectangular shaped cutting blade 48. It will be appreciated that a rotor assembly performs three functions in countercurrent mixing: that is, liquid mix ing, dough chopping and granulation of the product. Another rotor assembly which is particularly well adapted to perform these functions is shown in FIG. 12. Attached to the underside of plate 49 is a series of rather short pins 50 which are mounted about the perimeter of plate 49. These pins are designed to help promote mix ing of the reactants while they are still in a liquid phase. Mounted to the top side of plate 49 are a series of longer pins 51 which are designed to help promote granulation of the soap mass. Mounted near the top of shaft 52 are a pair of generally rectangular shaped open box-like choppers 53 which are designed to rip or chop the heavy dough-like soap mass. It is desirable that chop pers 53 not contact the fluid reaction mass until it is semi-solid and non-sticky and therefore are mounted at a distance above the ends of pins 51. Referring again to FIG. 11 the mixing equipment is also provided with an air blowing system consisting of an air pressure blower system shown generally at 60 and exhaust means shown generally at 70. The air pressure blower system includes blower 61 driven by motor 62 and duct 63. The system may also be provided with a cooling means 64, such as an evaporative cooler which serves to cool the air. Air is drawn through cooling means 64 and then forced into the interior of mixer 30 via duct 63. It is also possible to provide heating means in place of or in addition to the cooling means 64. A steam heated heat exchanger would be quite suitable. The exhaust means 70 consists of suitable blower mounted in housing 71 powered by motor 72. A vertical pipe 73 is mounted to one end of the blower housing. Exhaust duct 74 is connected between the interior of mixer 30 and the upper end of pipe 73. OPERATION Referring to the equipment shown in FIGS. 1-3 in making soap from the customary raw materials the required amount of caustic can be introduced into the mixing equipment through the loading door 14. After the caustic is in the mixing pan 11 rotation of the pan is started and thereafter the fatty acid source is charged into the mixing pan either through the loading door 14 or preferably through port 15. Rotation of rotor assem bly 18 is begun and the intensive countercurrent mixing of caustic and fatty acid takes place. The head on meet ing of the rapidly moving stream of caustic and fatty acid source and the counter rotating assembly 18 cre ates a generally rotary movement of the reactants within the mixing pan appearing much like an eddy stream or whirlpool as shown by dotted arrow 29a of FIG. 2. As processing proceeds the reactants, which are initially in the liquid phase, gradually form a viscous,
8 7 grainy appearing mass resembling mashed potatoes and it is at this stage that air may be introduced into the mixing pan through port 16 to enhance formation of soap granules. Continued mixing results in a more vis cous dough-like mass which, upon continued mixing, start to pull apart and shred into taffy-like strands and eventually breaks down into non-tacky granules. While the mixing is underway additives normally employed in soap making such as brine solution, chelat ing agents, glycerine, and the like can be introduced into the mixing pan via port 15. As previously noted the mixing equipment shown generally at 30 of FIG. 11 is able to handle much larger batches than the equipment of FIGS Although not shown, the mixer 30 is provided with suitable piping which permits introduction of the various soapmaking materials such as the triglycerides, fatty acids, caustic, and other additives directly into the interior of the mixer. In practicing our process the fatty acid sources em ployed may be any of those which are customarily used in the making of soap. The limitation on the types of fatty acid sources employed is therefore dependent only on the particular qualities of the soap which are desired. Where fatty acids are readily available such acids rang ing in chain length from 6 to 18 are usually employed. Aqueous sodium hydroxide is commonly used to saponify the fatty acids or triglycerides, although aque ous potassium hydroxide can be used in the preparation of a so-called softer soap since the potassium soaps are more water soluble than the sodium soaps. It is also possible to use blends of the two alkalis in order to achieve special properties. The amount of caustic employed in our process is that which is theoretically necessary to completely saponify the fatty acid source excepting in the production of so-called superfatted soap where the fatty acid source would be in excess. The caustic should be in liquid form and depending on the desired moisture content of the soap granules, will be at a concentration ranging from 10% to 70%, with the optimum being about 50%. It is possible to use 100% caustic when using ingredients which are high in moisture such as an alpha olefin sulfo nate solution which normally contains about 70% by weight of moisture. The temperature of the caustic solution will normally range from about 1 F. to about 210 F. The amount of water present in the caus tic and other additives customarily used in soap prod ucts will affect the amount of time required to reach the desired moisture level in the final soap granules. In addition, the saponification reaction itself produces some water as shown by the following: Fatty Acid-Caustic Soda=Soap-i-Water R-COOH--NaOH =R-COONa+ H2O. Thus, according to the foregoing in theory approxi mately 6% by weight of water will be obtained in the reaction. This of course does not take into account water which is lost through the heat generated by the reaction, which we have found to usually be about 3% by weight of soap produced. As with the caustic, the fatty acids and/or triglycer ides are preferably liquid and at a temperature ranging from the melting point of the fatty acids or triglycerides to about 170 F. Although the reaction takes place somewhat more rapidly when these materials are at higher temperatures, because fatty acid sources at the lower temperatures give satisfactory results and are more easily handled, the optimum temperature range is from the melting point of such sources to about 140 F. In reacting the fatty acid sources and caustic through the use of intensive countercurrent mixing, it is also possible and even desirable to incorporate into the mix ture other ingredients that are customarily found in soap products, such as perfumes, colorants, emollients and the like. It is preferable that these additional materi als be added to the mixing vessel after the saponification reaction has proceeded for a period of time. In subjecting the fatty acid sources and caustic to intensive countercurrent mixing, we find that the intro duction of air into the mixing vessel during saponifica tion greatly enhances the formation of soap granules. The introduction of air not only minimizes processing time and energy requirements but, in addition, serves to reduce the moisture level and lower the temperature of the reaction mixture which also helps to preserve heat sensitive ingredients. Although most fatty acids and triglycerides and caustic are basically not heat sensitive, other additives which may be included in the mixture, such as perfumes and oxidation inhibitors, are, and the blowing of air into the reaction vessel does serve to protect such ingredients. It is preferable to introduce the air when the saponification reaction is essentially complete. For a mixing pan having a capacity of about 100 pounds we find that air delivery of from 160 to about 250 SCFM works satisfactorily. With a mixing vessel having a capacity of about 800 pounds air deliv ery of from 1000 to 10 SCFM worked satisfactorily. The air may be introduced directly into the mixing pan through port 16 as shown in FIG, 1 or port of FIG. 11. The order of addition of the principal reactants in our process does seem to affect the quality of the end prod uct and can vary depending upon the batch size. Al though acceptable soap granules are formed with virtu ally any order of addition, the preferred procedure with a mixing vessel as shown in FIGS. 1-3 is to charge the liquid caustic into the mixing vessel followed by a start up of the rotating pan. The fatty acids are then charged into the vessel over about a 2 minute period and thereaf ter the rotor is started. After the intensive countercur rent mixing has proceeded for a period of time, the free caustic level of the soap can be adjusted by adding either additional caustic or fatty acids. When it has been determined that neutralization is essentially completed, air is introduced to cool the mixture and to help remove moisture. The moisture level can be determined by appropriate measuring instruments. The intensive coun tercurrent mixing can be continued until the soap be comes a powder and has a moisture content of from 3-8%. A preferred order of addition when using a vessel having a capacity of about 100 pounds is: a. Charge caustic solution into vessel. b. Begin rotation of pan. c. Charge fatty acid and/or triglyceride into vessel. d. Start rotor assembly. e. Add brine solution with other additives such as che lating agents, glycerine, silicates and the like. f. Continue mixing until saponification is essentially complete. g. Begin blowing of air into vessel while continuing the intensive countercurrent mixing.
9 h. Continue drying and mixing until granules form and the desired moisture level is reached. i. Add soap slurry and perfume; these are ingredients normally added to the amalgamator in a conventional soap making process. j. Discharge when slurry and perfume are completely mixed-usually about 1 minute. FIGS. 4 and 5 shows different types of mixing tools which may be employed in the mixing equipment de scribed herein and as shown in FIG. 1 and FIG. 2. There is no significant difference in the mixing abilities of these tools, although the mixing patterns are some what different. The star rotor shown in FIG. 5 splashed the mixture somewhat which was not a problem when the pin mixing tool of FIG. 4 was used. Therefore the pin-style mixing tool is preferred. When using a larger mixing vessel such as is shown in FIG. 11 which has a capacity of about 800 pounds, the most preferred procedure is to initially introduce at least a portion of the fatty acid source and then begin to introduce the caustic. Thus a preferred order of addi tion when utilizing the mixing vessel shown in FIG. 11 is as follows: a. Begin feed of fatty acid source. b. Begin feed of caustic when about of the fatty acid source is in the vessel. c. Start rotation of pan, mixing tool and high speed rotor. d. Begin addition of any additives when about of the 30. caustic is in the vessel. e. Continue mixing until saponification is essentially complete. f. Begin addition of air while continuing the intensive counter-current mixing. g. Remove granules when desired moisture level is obtained. When processing neat soap, the neat soap is intro duced into the vessel and rotation of the pan is begun. Thereafter the rotor assembly is started and brine solu tion and other additives such as chelating agents, glyc erine, silicate and the like may be added. It is also possi ble to blend these additives into the neat soap prior to its introduction into the mixer. Blowing of air into the mixing vessel is started while continuing the intensive counter current mixing. When the desired moisture level is achieved, a soap slurry and perfume can be added and the resulting soap in granular or powder form is discharged when the slurry and perfume are completely mixed into the soap mass. The pan speeds and rotor speeds employed are substantially the same as when starting with an unreacted fatty acid source and caustic. It should also be noted that neutral soap gran ules containing none of the above additives can be pro duced by our mixing technique. Such additives may be included in the granules at a later stage, In Examples I and IX which demonstrate the versatil ity of our process all the processing was conducted in a Model R-7 Eirich Mixer manufactured by Maschinen fabrik Gustav Eirich of Nordbaden, West Germany. This mixer has a capacity of about 2 cubic feet, with a batch size of about 100 pounds. We find that pan speeds of rpm, preferably about 25 rpm, and rotor speeds of from 100 to 2400 rpm, preferably about rpm, are sufficient to provide low moisture soap gran ules. With this size mixing equipment, an air flow of up to about 250 SCFM gave the desired results, EXAMPLE I A sodium stearate soap was made using the follow ing: Stearic Acid NaOH (50% solution) Additives (glycerine, chelating agent, preservative) Brine (6% solids) solution 700 lbs..9 lbs. 3.3 lbs. 3.2 lbs. The sodium stearate soap was prepared according to the following steps: 1. The caustic was added to the mixer pan. 2. Rotation of the mixer pan was begun at 48 rpm. 3. The fatty acid was charged into the mixing pan through port 15 over a period of two minutes. 4. The rotor assembly was turned on at 700 rpm. 5. The brine and additives were charged into the mixing pan. 6. The speed of the rotor was increased to 1400 rpm. 7. Mixing was continued for a period of approximately ten minutes. 8. Air was introduced into the mixing pan while con tinuing the intensive countercurrent mixing at 0 cfm for a period of minutes. 9. The sodium stearate soap granules were removed from the mixing pan. Analysis indicated a moisture content of approximately 12 percent. EXAMPLE II A superfatted soap base was prepared from the fol lowing ingredients: Tallow/Coco Fatty Acids, 70:30 ratio Sodium Hydroxide (50% solution) Additives (glycerine, chelating agent, silicate) Brine solution (6% Solids) Coco Fatty Acid 700 lbs, lbs bs lbs lbs. In preparing the superfatted soap base, the sodium hydroxide was introduced into the mixing pan and rota tion of the pan was begun at 48 rpm. Thereafter the tallow/coco fatty acid blend was added to mixing pan through one of the ports over a period of 2 minutes followed by start-up of the rotor at a speed of 1400 rpm. After a period of 2 minutes the brine and additives were introduced into the mixing pan and the intensive coun tercurrent mixing was contained for a period of 2 min utes. After this the coco fatty acid was added to the mixture and air was introduced into the mixing pan at 0 cfm. for a period of 24 minutes. The soap base in granular form was removed and analysis showed that it had a moisture content of 11 percent, EXAMPLE II A 70:30 ratio tallow/coco soap base was prepared as follows: Tallow/Coco Fatty Acids, 70:30 ratio Sodium Hydroxide (50% solution) Additive (glycerine, chelating agent, silicate) Brine (NaCl, 6% solids) 700 lbs, 22.5 lbs, 3.9 lbs, 3.4 lbs, The caustic was introduced into the mixing pan and rotation of the pan was begun at 48 rpm. The fatty acids were added over a period of 2 minutes via one of the ports and the rotor assembly was started at 1400 rpm,
10 11 Thereafter the brine and additives were charged into the pan and the intensive countercurent mixing contin ued for minutes. Air was then introduced into the mixing pan at 180 cfm, and mixing was continued for an additional 15 minutes. Mixing was then discontinued and soap granules having a moisture content of about 12% and a diameter averaging about $ inch were ob tained. EXAMPLE IV A perfumed soap base was prepared according to the following: Tallow/Coco Fatty Acids, 85:15 ratio... Sodium Hydroxide (50% solution) Brine (NaCl- 6% solids) Additives (Chelating agent, glycerin, silicate) Slurry (colorants, antioxidants) Perfume 700 lbs lbs. 4.8 lbs. 1.7 lbs. 2.3 lbs lbs. In preparing the soap, the sodium hydroxide was introduced into the mixing pan and rotation of the pan was begun at 48 rpm. Thereafter the tallow/coco fatty acid blend was added to mixing panthrough one of the ports over a period of 2 minutes followed by start-up of the rotor at a speed of 1400 rpm. After a period of 2 minutes the brine and additives were introduced into the mixing pan and the intensive countercurrent mixing was continued for a period of 15 minutes. Air was then introduced into the pan while mixing continued at 180 cfm and for a period of minutes. The air was shut off and thereafter the slurry and perfume were introduced in the pan followed by additional mixing for 1 minute. The soap granules were removed and analysis indicated a moisture content of 12%. EXAMPLE V A tallow/coco fatty acid soap was prepared from the following materials.. Tallow/coco fatty acid (65:) Caustic (50% solution of NaOH) 70 lbs lbs.. Brine (6% solids) -. Additive (glycerin, chelating agent and water) 448 lbs lbs. The foregoing were subjected to intensive counter current mixing according to following procedures. O Rotor Pan -continued Time Speed Speed (minutes) (rpm) (rpm) Action/Observation ment) 42 Stop Stop Soap observed to be large "popcorn' size granules Start 48 Stop Stop Stop - remove soap granules temp = 185 F. temp = 184 F. The moisture content of the soap removed after 48 minutes of processing was 11% and the soap was at a temperature of 1 F. EXAMPLE VI A tallow/coco fatty acid soap was prepared from the following materials. Tallow/coco fatty acid (70:30) Caustic (50% solution of NaOH) Brine (7% solids) Coco fatty acid Additive (glycerin, chelating agent, water) 70 lbs lbs lbs lbs lbs. The foregoing maerials were subjected to intensive countercurrent mixing according to the following pro cedures. Rotor Pan Time Speed Speed (minutes) (rpm) (rpm) Action/Observation Add caustic (168 F) Add tallow/coco fatty acid (190 F) Add brine : Introduce additive 2 Phase change; doughlike consistency 3 Stripped coco F.A. added; back to liquid state 3. Introduce air at 0 cfm; phase change to dough like consistency 7 Stop Stop Scrape pan wall Start up again 10 Stop Stop. Granulation beginning Start up again 23 Virtually all soap formed into gran ules 24 Reaction complete; granules range in size from " to 1". Moisture = 11% Rotor Pan Time Speed Speed (minutes) (rpm) (rpm) Action/Observation - Added caustic 48 Start pan rotation and Start addition of fatty acid Complete addition of fatty acid, l 700 Start rotor 2 Add brine 2 Introduce additives 4. Phase change in mixture noted 4. Stop Stop Stopped to scrape pan wall Start 10 Stop Stop Stop for soap sample Start 15 Stop Stop Stop for soap sample Start 18 Stop Stop Stop to scrape pan wall Start - air introduced, 0 cfm 33 Added 100 ml, fatty acid (ph adjust ment). 36 Added 25 mi. 32% NaOH (ph adjust EXAMPLE VII Transparent soaps are usually made by a so-called semiboiled method followed by framing with substan tial quantities of alcohol, glycerine or sugars included in the soap to promote a glossy, transparent condition. For example, commercial transparent bar soap is normally made by charging the fatty acid, triethanolamine, and sodium hydroxide into a kettle and boiling for several hours at 1 C., along with the addition of glycerine to inhibit growth of soap crystallites during subsequent framing and to promote transparency. After saponifica tion is completed, the soap is poured into frames where the soap cools and solidifies. To achieve a desirable moisture level of about 10 to 12 percent the soap must be left in the frame for a period of up to 60 days. To demonstate the use of the intensive countercur rent mixing process in the preparation of a transparent soap base, the following ingredients were prepared:
11 NaOH NaCl Water Fatty Acid Mixture: Stearic Tallow Cs/C10 Oleic Ricinoleic Glycerine: Triethanolamine: lbs lbs/ 6.0 lbs lbs lbs. 2.6 lbs. 1.0 lbs. 3.0 lbs. 100 lbs, 300 lbs. In processing, the caustic mixture was introduced into the mixing pan and rotation of the pan was begun at 48 rpm. Thereafter the fatty acid mixture was added through one of the ports and the rotor assembly was started at approximately 700 rpm. The triethanolamine and glycerine were added and the speed of the rotor was increased to 1400 rpm. Air was introduced into the mixing pan at about 0 cfm and the intensive mixing was continued for a period of about one hour. The resulting product was somewhat runny and plastic like, aerated and melted at 140 F. The batch was removed from the mixer and placed in a steam jacketed kettle and melted. Some foam formed on the surface of the liquid and was skimmed off. The liquid soap was then poured into trays and after a period of about 2 hours was hard enough to cut with a knife. The cut pieces of soap were slightly filmy but became virtually crystal clear when wet. Analysis of the soap gave the following results: Sodium Soap 30.5 weight percent TEA Soap 25.9 weight percent Free TEA 22.1 percent Water 6.3 percent ph 8.4 After approximately two days exposure to air the bars became dry to the touch. EXAMPLE VIII The versatility of the intensive countercurrent pro cess can be demonstrated in the preparation of a soa p-alpha olefin sulfonate product. The following ingre dients were placed into separate containers. Container A Alpha olefin sulfonate (C14-C18,30% active) Sodium lauryl sulfate Silicate Glycerin Chelating agent Container B. Fatty alcohol (Adol 63) Stearic acid Paraffin (high MP) Amide Polyethylene glycol (PEG 6000) Cocoamide (All of the foregoing in B were heated to 150 F) Container C Sodium hydroxide pellets Container D Tallow/Coco fatty acid (85:15) Coco fatty acid (hydrogenated) (All of the foregoing in D were heated to 150 F) 30 lbs bs lbs lbs lbs lbs lbs lbs lbs lbs lbs. 6,68 bs lbs lbs In preparing the soap/synthetic product by intensive countercurrent mixing the following steps were fol lowed. 1. The contents of container A were poured into the mixing pan and pan rotation begun at 48 rpm. 2. After approximatly 30 seconds the NaOH pellets were added and mixing by rotation of the pan only was continued for about 1 minute. 3. Thereafter the fatty acids (Container D) were intro duced and the rotor assembly started at 1090 rpm. Mixing was continued for about 10 minutes. 4. The contents of container B were then added and mixing was continued for a period of about 85 min utes. 5. Mixing was stopped and the product was observed to be non-tacky granules having a diameter of about 1'. These granules were subsequently plodded, extruded and stamped into bars. EXAMPLE X A series of experiments were run to determine the effect of blowing air into the mixing pan after saponifi cation was essentially complete. In all these experiments the following formula was used. Tallow/Coco Fatty Acids 85/15 70 lbs. NaOH (50%) bs. Additives 2.28 lbs. Brine (6% solids) 2.13 lbs. Slurry 2.28 lbs. Perfume 0.65 lbs. Also, in each of the experiments the following proce dure was followed: (1) Charge caustic solution into mixing pan. (2) Start pan rotation, 48 rpm. (3) Charge fatty acid over a 2 minute period. (4) Start rotor assembly, 1090 rpm. (5) Add Additives and Brine. (6) Mix for 5 minutes. (7) Begin air blowing. (8) Add Slurry and Perfume. (9) Continue mixing for 15 minutes with a stopping of mixing every 2 minutes for samples. (10) Discharge soap granules. In evaluating the effect of air blowing, the soap was sampled every 2 minutes and temperature and moisture determinations were made. The following results were obtained: Air Blow Rate Drying Rate Cooling Rate 0 cfm.47% H2O/min. 3.3 F.Amin. 160 cfm.% H2O/min. 1.6 F.Amin O cfm.16% Hovnin. O With no air blow the cooling rate of saponification product is negligible and under these conditions a high temperature is maintained which may adversely affect heat sensitive ingredients such as perfume. It should also be noted that with air blow the drying rate of the saponification product was substantially increased which reduces processing time and energy require ments. In the following Examples X, XI and XII processing was conducted in the larger Model DE-14 Eirich Mixer. This mixer, which is shown in FIG. 11, employs a horizontal rotating pan approximately 1400 mm in
12 15 diameter and has a batch size of from about pounds. As shown in FIG. 11 the mixer is provided with a rotating mixing tool 38 with kneading bar 39 and mixing plow 40. Mixing tool 38 is mounted eccentri Start drying by blowing air into reaction vessel. 10. Discharge granules when at temperature of about 115 F. The results of the tests are as follows. RUNNO (4 5 TEM UNITS cally in the pan and rotates in a direction opposite to that of the rotation of pan 31 and during the tests was run at 52 rpm. Also eccentrically mounted in pan 31 is high speed rotor 42 which also rotates in a direction 40 opposite to that of the pan. The rotor speeds employed in the tests were 626 and 1253 rpm. The pan speed in all tests was 113 rpm. The mixer was also equipped with an air blowing system consisting of a 7.5 hp blower 61 and a 15 hp exhaust blower 71 provided with a blast gate, not shown, designed to control the suction from the exhaust fan. Air flows of from 1000 to 10 SCFM were employed. In addition a 5000 CFPM evaporative cooler was installed in the air stream to permit control of the temperature and humidity of the air being circulated in 50 the mixer. In addition a steam heated radiator, not shown, was also installed in the air stream to permit heating of the air stream when desired. EXAMPLE X A series of runs was made using the following proce dure, unless otherwise noted in the charts below. 1. Charge fatty acid; a 75:25 ratio of tallow to coco was used in all runs except number Start pan and mixer (11 and 52 rpm respectively) Start rotor (626 rpm). 4. Add 50% caustic solution feeding at about 25 lbs./mi nute. 5. Start addition of glycerin solution, where used, when about one-half of caustic has been added Charge remaining caustic at lbs/minute. 7. Mix for about 15 minutes at rotor speed of 626 rpm. 8. Check alkalinity and adjust if necessary. Total Time Alkali in Minutes % Neutral Prod. 75T/25C lbs/f. 485/ /1 480/52 481/134 85T/15C lbs/f /126 Caustic lb/f, 93.7/77 52/ /8/ / /167 12% Brine bs/f. 5.4/ 24.4/1 24.4/ 24.4/1 Glycerin lbs/f. 17.4/ / /1 Total lbs ,8 672,8 Drum/Mixer RPM 1 1/52 11/52 11/52 1/52 11/52 Rotor RPM 626/ / / / In Fan RPM (977 SCFM) Exhaust Fan RPM OFF OFF (1085 SCFM) Cooler On/OFF Air Heat On/Off OFF OFF OFF OFF OFF On 67 min, ON OFF ON OFF Order of Addition 1 Caustic Caustic & F.A. F.A. F.A. F.A. 2 3 F.A. Brine Brine Caustic Glycerin Caustic Glycerin Caustic Glycerin 4. Brine Brine Initial F Charge Maximum F Granule type/f. /150 Fine/129.9 Med/137.9 / 16.5 Med../1.6 Comments Homogen- 2 plows High % Glycerin eous in used. Some Caustic added when 22 min. hard lumps helped of caustic of caustic granula- in. Homo Soap tion geneous in 14 min. In analyzing the foregoing runs, it should be kept in mind that the processing of the reactants into granules involves three basic steps, that is: the mixing of the reactants to homogeneity, a drying step, and finally granulation. It should be noted in these series of runs that the reactant addition sequence is somewhat differ ent from that shown in Examples I-IX. It was found that adding the caustic first to the mixing vessel caused the formation of some hard, high alkalinity particles. which were difficult to break up and disperse in the subsequent mixing stages. In addition, adding both reac tants together also formed some of these high alkalinity particles. It was found to be perferable to first introduce the fatty acid. In addition we found it is desirable to permit the reaction temperatures to reach F. and when the reaction heat did not reach at least about 190 F., some high alkalinity hard particles were formed. It was also observed that control of alkalinity is important. A high alkalinity, that is greater than about 0.1% of caustic, gave a more viscous neat soap which appeared much dryer than it actually was. When the mix was slightly on the acid side, fluidity was better and the time to homogeneity was reduced considerably. With respect to the matter of the drying step, it was noted that the blowing of air through the reaction vessel was important in achieving reasonable processing times. An air velocity of SCFM gave good results in lowering the batch temperature. It is possible that even greater air flow rates may be advisable with the limiting factor being the point where some reaction product could be carried over into the exhaust system. In the foregoing runs it was also observed that the air
13 17 temperature had a direct affect on the granule moisture and this effect can be summarized as follows: Temperature of Incoming Air Granule Moisture ' F % F. 8-12% Greater than 100' F. 7-8% The general parameters that appear to have an affect 10 on the moisture level of the granules and processing time are: a. Starting formula moisture level. Increasing this mois ture level increases granule moisture and also in creases processing time. b. The rate of air blow; that is, increasing the rate of air blow increases the rate of temperature loss resulting in granules having a higher moisture content and correspondingly results in shorter processing times. c. As the temperature of the air being blown into the reaction vessel is lowered, a corresponding increase in the moisture content of the final appropriately sized granules and a decrease in the processing time was observed. d. Reaction batch temperatures in the range of F.-210 F. appear to give optimum results. EXAMPLE X A further series of runs in the intensive mixing equip ment described in Example X was conducted using the 50 formula types as shown below The purpose of these tests was to determine the effect of increasing the moisture content of the reactants somewhat, that is up to about 2% by weight; the effect of decreasing the feed temperatures of the reactants; and the effect of increasing reactant load in the mixing vessel. In conducting these tests the following proce dure was followed: 1. Start feed of fatty acid. 2. Start feed of caustic when about of fatty acid had been introduced into mixer. 3. Begin rotation of pan (11 rpm) and rotor (626 rpm). 4. Begin introduction of additive when about one-half of caustic is in. 5. Begin timing when all caustic is in mixer. 6. At about 7 minutes open dampers and start pressure and exhaust blowers. 7. Adjust alkalinity if required. 8. Five minutes later again adjust alkalinity if required. 9. Remove granules when following conditions occur: Formula Type F. A 116 B 115 C 114 D 113 E 112 The following results are typical for the various tests. RUNNO FORMULATYPE A B C D E Batch charge Lbs. 75/25 T/C/F. 8.5/ /128 7/122 5/122 6/128 NaOH/F /40 77/161 77/ /143 77/142 Additive/"F. 79.5/ / / / /80 Extra H2O lbs Slurry 9.5 Perfume 16.5 Total lbs S % H2O after Neutralization Rotor RPM Chiled Air YES YES YES YES YES Air in "F Peak Temperature Discharge Temperature Total Time (Minutes) Analysis of Granules 69% 78.6% %. 1" size It was observed that by increasing the moisture level of the batch charge a reduction in granule size was A B C % % % Base Formula. 75/25 T/C Fatty Acid (222 A.V.) NaOH (50%) Additive (NaCl, preservatives, etc.) Extra H2O O Slurry (Colorants, H2O, TiO2, 0 O O Dispersants) Perfume O O Theoretical: Total H2O % (after saponification) Total Charge (bs.) Total Product (theoretical with 12% H2O) D E % % O ,
14 19 obtained. In addition, the granules were more uniform in size and concentrated in the 8' to 1" range. However, this additional moisture also generally increased pro cessing time. These tests also showed that a decrease in the temperature of the reactants did not adversely affect granulation so long as the peak temperature of the reac tion got up to about F. It was also learned that the fatty acid and caustic could be introduced al most simultaneously as long as sufficient fatty acid was added to make contact with the pins of the rotor and that the addition of caustic extended beyond the addi tion of the fatty acids. EXAMPLE XII Fats and Oils Saponification Utilizing the equipment described in Example X soap granules were prepared from tallow and coconut oil. The tallow and coconut oil were heated to 150 F. and 510 lbs. of tallow and 90 lbs. of coconut oil were intro duced simultaneously in the intensive mixer. A stoichio metric amount of 50% NaOH was then introduced into the mixer over a period of about 90 minutes. The pan speed was 11 RPM, the mixing tool was run at 52 RPM and the high speed rotor run at 626 RPM. After a total reaction time of 130 minutes saponification was 99.3% complete. An analysis of the soap granules indi cated the following: 10.5% moisture. 8.5% glycerine. Light brown color. ' size granules (average). EXAMPLE XII The mixer shown in FIG. 6 is a Hobart Model A0 mixer and is shown generally at 80. The mixer consists of housing 81 with a two speed motor (not shown) in the upper portion 87 of the housing. Mounted between legs 88 and the housing is a stationary bowl 82. A pad dle-shaped mixing tool 83 with attendant shaft 84 is secured in chuck 85 which in turn is attached to gear housing 86. Mixer 80 is designed so that the mixing tool 83 rotates in a counter-clockwise direction while at the same time is following an orbital clockwise path around the interior of bowl 82. This sets up a counter-current mixing process. Thus the gear housing will rotate in a clockwise direction while the tool 83 rotates in a coun ter-clockwise direction. -- Soap granules were prepared in the mixer using the following materials. ' ' '... Tallow/coco fatty acid (70:30) Caustic (50% solution of NaOH) Brine (6% solids) 10 lbs. 3.2 lbs lbs. The fatty acid blend was charged into bowl 82 at a temperature of about 130 F. The brine and caustic solution were combined and added to the bowl at room temperature over a period of about 1 minute. The mix ing tool 83 was set to rotate counter-clockwise at 115 rpm and its orbit was 47 rpm in a clockwise direction. In approximately 10 minutes the batch went from a thin milky liquid to a dough-like state. At this point ambient air was directed at the batch by means of a blower and mixing was continued. After about twenty minutes of 65 further mixing granulation tool place. The resulting product was randomly sized granules with average size about inch. This demonstrates that the process of high intensity countercurrent mixing is very important in providing soap granules and also demonstrates that a wide variety of equipment may be employed in our process. From the foregoing it is apparent that intensive coun tercurrent mixing as described herein provides a supe rior technique for rapidly producing a low moisture soap in granular form under ambient pressure condi tions. EXAMPLE XIV 4000 grams of neat soap containing as additives, glyc erine and a resin was processed by intensive countercur rent mixing in a Model RO2 Mixer available from Ei rich Machines Ltd. Although this mixer is of considera bly smaller capacity than the mixers used in other of the examples, having a capacity of about 10 liters, it oper ates in the very same manner as the equipment shown generally at 10 and 30. The composition of the neat soap is as follows: Neat Soap (Tallow/coco fatty acid ratio 65.) containing 31.5% water by weight Glycerin Resin 3864 gms. 56 gms, 80 gms. After the neat soap was introduced into the mixer, rotation of the pan was begun at 33 rpm with a rotor speed of 7 rpm. Air at ambient temperature was intro duced at about 83 cfm. Mixing was continued for a period of minutes at which time the mixing was discontinued and 'pea' size soap granules were ob tained. The granular soap was then run through a Maz zoni finishing plodder, extruded and stamped into bars. We claim: 1. A process for making soap granules from a mixture of a fatty acid source and caustic or alkali wherein. neutralization of said fatty acid source has proceeded to some degree comprising the steps of introducing said mixture into an enclosed mixing vessel, causing said mixture in said vessel to rotate in a generally circular path while simultaneously bringing said mixture into contact with a rotating means mounted within said vessel, said means rapidly rotating in a direction counter to the initial direction of flow of said mixture in said vessel whereby saponification, if required, takes place and soap granules are formed having a moisture content of less than about % by weight. 2. A process according to claim 1 wherein said mix ture also includes a synthetic detergent. 3. The process according to claim 1 wherein air is blown into said vessel during mixing. 4. The process according to claim 1 wherein said fatty acid source are long chain monocarboxylic acids having a chain length of from 6 to 18 carbon atoms. 5. A process according to claim 1 where neutraliza tion of said fatty acid source is essentially completed prior to introduction into said mixing vessel. 6. A process according to claim 5 wherein said counter rotating means are mounted eccentrically within said vessel and at a distance from the wall of said vessel. 7. A process according to claim 5 wherein said soap granules are removed from said vessel and thereafter subjected to plodding, extrusion and stamping to form soap bars. k R k k k
Soap Fabrication. 1. Introduction [1]
![Soap Fabrication. 1. Introduction [1] Soap Fabrication. 1. Introduction [1]](/thumbs/82/86094217.jpg) 1. Introduction [1] Soap Fabrication The main uses of soap include bathing, washing, cleaning and other types of housekeeping. Soap acts as surfactant because it has surface active properties. When Soaps
1. Introduction [1] Soap Fabrication The main uses of soap include bathing, washing, cleaning and other types of housekeeping. Soap acts as surfactant because it has surface active properties. When Soaps
Saponification and the Making of Soap - An Example of Basic Catalyzed Hydrolysis of Esters
 1 of 5 9/7/2010 2:56 PM Experiment 8 Saponification and the Making of Soap - An Example of Basic Catalyzed Hydrolysis of Esters Objectives In today's experiment, we will perform a reaction that has been
1 of 5 9/7/2010 2:56 PM Experiment 8 Saponification and the Making of Soap - An Example of Basic Catalyzed Hydrolysis of Esters Objectives In today's experiment, we will perform a reaction that has been
Preparation and Properties of Soap Experiment #7
 Preparation and Properties of Soap Experiment #7 Objective: To prepare soap by alkaline hydrolysis (saponification) of natural fats and test some of the chemical properties and cleansing power of soap
Preparation and Properties of Soap Experiment #7 Objective: To prepare soap by alkaline hydrolysis (saponification) of natural fats and test some of the chemical properties and cleansing power of soap
zzºzzzzzzzzzzzzzzzz';
 Oct., 1973 R. G. MATTHAE PROCESS FOR PRODUCING MARBLEIZED SOAP Filled Feb. 12, 1971 2 Sheets-Sheet 1 2xxar as zzºzzzzzzzzzzzzzzzz'; Zzzzzzzzzzzzzzzzzzzzº RAYMOND GEORGE MATTHAE INVENTOR. re-3- ATTORNEYS
Oct., 1973 R. G. MATTHAE PROCESS FOR PRODUCING MARBLEIZED SOAP Filled Feb. 12, 1971 2 Sheets-Sheet 1 2xxar as zzºzzzzzzzzzzzzzzzz'; Zzzzzzzzzzzzzzzzzzzzº RAYMOND GEORGE MATTHAE INVENTOR. re-3- ATTORNEYS
SOAP. A brief discussion on the History, Base Construction, Formulating and Manufacturing
 SOAP A brief discussion on the History, Base Construction, Formulating and Manufacturing HISTORY Said to be oldest hand manufactured product by mankind Improved hygiene Reduced infection and disease Twincraft
SOAP A brief discussion on the History, Base Construction, Formulating and Manufacturing HISTORY Said to be oldest hand manufactured product by mankind Improved hygiene Reduced infection and disease Twincraft
Preparation and Properties of Soap
 Preparation and Properties of Soap Experiment #6 Objective: To prepare soap by alkaline hydrolysis (saponification) of natural fats and test some of the chemical properties and cleansing power of soap
Preparation and Properties of Soap Experiment #6 Objective: To prepare soap by alkaline hydrolysis (saponification) of natural fats and test some of the chemical properties and cleansing power of soap
(12) United States Patent (10) Patent No.: US 8.481,614 B2
 USOO8481.614B2 (12) United States Patent (10) Patent No.: US 8.481,614 B2 Mantzivis (45) Date of Patent: Jul. 9, 2013 (54) MASTERBATCH PREPARATION PROCESS (52) U.S. Cl. USPC... 523/351 (76) Inventor: Lionel
USOO8481.614B2 (12) United States Patent (10) Patent No.: US 8.481,614 B2 Mantzivis (45) Date of Patent: Jul. 9, 2013 (54) MASTERBATCH PREPARATION PROCESS (52) U.S. Cl. USPC... 523/351 (76) Inventor: Lionel
58 Field of Search ,151,152, coconut oil and about 15 wt % to about 35 wt % fatty acid.
 USOO6133225A United States Patent (19) 11 Patent Number: 6,133,225 Tokosh et al. (45) Date of Patent: *Oct. 17, 2000 54) SOAP BAR HAVING ARESISTANCE TO 4,147,053 4/1979 Marchesani... 73/104 CRACKING AND
USOO6133225A United States Patent (19) 11 Patent Number: 6,133,225 Tokosh et al. (45) Date of Patent: *Oct. 17, 2000 54) SOAP BAR HAVING ARESISTANCE TO 4,147,053 4/1979 Marchesani... 73/104 CRACKING AND
The Production of Natural Waxes Based Candles on Halfautomatic, Automatic and Continuously Working Molding Machines
 Spezial-Maschinenfabrik Hans Kürschner GmbH & Co. KG Postfach 22 20 D-41309 Nettetal (Kaldenkirchen) Federal Republic of Germany Telefon: (+ 49) (0)2157 1215-0 Fax: (+ 49) (0)2157 1215-99 e-mail: service@kuerschner.de
Spezial-Maschinenfabrik Hans Kürschner GmbH & Co. KG Postfach 22 20 D-41309 Nettetal (Kaldenkirchen) Federal Republic of Germany Telefon: (+ 49) (0)2157 1215-0 Fax: (+ 49) (0)2157 1215-99 e-mail: service@kuerschner.de
Transparency and Meltability in Hot-Process Soap
 Transparency and Meltability in Hot-Process Soap Kevin M. Dunn Spring 2012 $Revision: 1.4 $ 1 Previous Work Modern Soap Making, Thommssen and Kemp, 1937 Making Transparent Soap, Failor, 1997, 2000 How
Transparency and Meltability in Hot-Process Soap Kevin M. Dunn Spring 2012 $Revision: 1.4 $ 1 Previous Work Modern Soap Making, Thommssen and Kemp, 1937 Making Transparent Soap, Failor, 1997, 2000 How
Experiment 13 Preparation of Soap
 Experiment 13 Preparation of Soap Soaps are carboxylate salts with very long hydrocarbon chains. Soap can be made from the base hydrolysis of a fat or an oil. This hydrolysis is called saponification,
Experiment 13 Preparation of Soap Soaps are carboxylate salts with very long hydrocarbon chains. Soap can be made from the base hydrolysis of a fat or an oil. This hydrolysis is called saponification,
CH 2 OH CHOH. gycerol
 Synthesis and Environmental Impact of Soap and Detergents Your group has been sent to a remote mountain region where life is still primitive. Here, the people still use traditional lye soaps, which have
Synthesis and Environmental Impact of Soap and Detergents Your group has been sent to a remote mountain region where life is still primitive. Here, the people still use traditional lye soaps, which have
BOOK V CHAPTER PART TWO SAPONIFICATION WITH RESPECT TO BASES THAT CAN FORM SALTS CHAPTER 4
 BOOK V CHAPTER 4 271 PART TWO SAPONIFICATION WITH RESPECT TO BASES THAT CAN FORM SALTS CHAPTER 4 THE EFFECT ON LARD OF SEVERAL BASES THAT CAN FORM SALTS 1011. The fat used for the following experiments
BOOK V CHAPTER 4 271 PART TWO SAPONIFICATION WITH RESPECT TO BASES THAT CAN FORM SALTS CHAPTER 4 THE EFFECT ON LARD OF SEVERAL BASES THAT CAN FORM SALTS 1011. The fat used for the following experiments
Romano et al. [45] Date of Patent: May 12, 1998
![Romano et al. [45] Date of Patent: May 12, 1998 Romano et al. [45] Date of Patent: May 12, 1998](/thumbs/94/120092598.jpg) 1111111111111111111111111111111111111111111111111111111I1111111111111111111 US005750202A United States Patent [19] [11] Patent Number: 5,750,202 Romano et al. [45] Date of Patent: May 12, 1998 [54] PREPARATION
1111111111111111111111111111111111111111111111111111111I1111111111111111111 US005750202A United States Patent [19] [11] Patent Number: 5,750,202 Romano et al. [45] Date of Patent: May 12, 1998 [54] PREPARATION
United States Patent Office
 United States Patent Office Patented Nov. 3, 1964 S8AP-MAKENG PERCCESS AND PRODUCT Williana A. Keilly, Teataeck, N.S., assign or to Lever Brother's Coapa Bay, New York, N.Y., a corporation of Maine No
United States Patent Office Patented Nov. 3, 1964 S8AP-MAKENG PERCCESS AND PRODUCT Williana A. Keilly, Teataeck, N.S., assign or to Lever Brother's Coapa Bay, New York, N.Y., a corporation of Maine No
BASICS OF HERBALISM 10 Alyse Rothrock 2007
 BASICS OF HERBALISM 10 Simple Syrup A simple syrup is a mixture of sugar and water.it can be used to deliver a tincture or unpleasent herbal blend. Useful for children. In making a simple syrup the key
BASICS OF HERBALISM 10 Simple Syrup A simple syrup is a mixture of sugar and water.it can be used to deliver a tincture or unpleasent herbal blend. Useful for children. In making a simple syrup the key
United States Patent (19) Putman
 United States Patent (19) Putman 11 Patent Number: 45 Date of Patent: Sep. 4, 1990 54. RHEOMETER DIE ASSEMBLY 76 Inventor: John B. Putman, 4.638 Commodore Dr., Stow, Ohio 44224 21 Appl. No.: 416,025 22
United States Patent (19) Putman 11 Patent Number: 45 Date of Patent: Sep. 4, 1990 54. RHEOMETER DIE ASSEMBLY 76 Inventor: John B. Putman, 4.638 Commodore Dr., Stow, Ohio 44224 21 Appl. No.: 416,025 22
Warp length compensator for a triaxial weaving machine
 United States Patent: 4,170,249 2/15/03 8:18 AM ( 1 of 1 ) United States Patent 4,170,249 Trost October 9, 1979 Warp length compensator for a triaxial weaving machine Abstract A fixed cam located between
United States Patent: 4,170,249 2/15/03 8:18 AM ( 1 of 1 ) United States Patent 4,170,249 Trost October 9, 1979 Warp length compensator for a triaxial weaving machine Abstract A fixed cam located between
(12) United States Patent (10) Patent No.: US 6,616,442 B2
 USOO6616442B2 (12) United States Patent (10) Patent No.: Venizelos et al. (45) Date of Patent: Sep. 9, 2003 (54) LOW NO PREMIX BURNER APPARATUS 5,201,650 A 4/1993 Johnson... 431/9 AND METHODS 5,238,395
USOO6616442B2 (12) United States Patent (10) Patent No.: Venizelos et al. (45) Date of Patent: Sep. 9, 2003 (54) LOW NO PREMIX BURNER APPARATUS 5,201,650 A 4/1993 Johnson... 431/9 AND METHODS 5,238,395
United States Patent (19)
 United States Patent (19) Marchesani 54 CRACK ELIMINATION IN SOAP 75) Inventor: Cesare N, Marchesani, Maywood, N.J. 73) Assignee: Colgate-Palmolive Company, New York, N.Y. (21) Appl. No.: 488,509 (22 Filed:
United States Patent (19) Marchesani 54 CRACK ELIMINATION IN SOAP 75) Inventor: Cesare N, Marchesani, Maywood, N.J. 73) Assignee: Colgate-Palmolive Company, New York, N.Y. (21) Appl. No.: 488,509 (22 Filed:
United States Patent (19)
 United States Patent (19) Yoshida et al. 54 SHAFT WITH GROOVES FOR DYNAMIC PRESSURE GENERATION AND MOTOR EMPLOYNG THE SAME 75 Inventors: Fumio Yoshida, Toride; Mikio Nakasugi, Chofu, both of Japan 73)
United States Patent (19) Yoshida et al. 54 SHAFT WITH GROOVES FOR DYNAMIC PRESSURE GENERATION AND MOTOR EMPLOYNG THE SAME 75 Inventors: Fumio Yoshida, Toride; Mikio Nakasugi, Chofu, both of Japan 73)
United States Patent (19) (11) 4,185,925
 United States Patent (19) (11) Gazzoni (45) Jan. 29, 1980 (54) SMALLSIZED TAPERED-END PLASTICS SILO, ESPECIALLY MATERAL FOR FOREIGN PATENT DOCUMENTS 1208570 9/1959 France... 366/319 75 Inventor I tor:
United States Patent (19) (11) Gazzoni (45) Jan. 29, 1980 (54) SMALLSIZED TAPERED-END PLASTICS SILO, ESPECIALLY MATERAL FOR FOREIGN PATENT DOCUMENTS 1208570 9/1959 France... 366/319 75 Inventor I tor:
(12) United States Patent (10) Patent No.: US 6,920,822 B2
 USOO6920822B2 (12) United States Patent (10) Patent No.: Finan (45) Date of Patent: Jul. 26, 2005 (54) DIGITAL CAN DECORATING APPARATUS 5,186,100 A 2/1993 Turturro et al. 5,677.719 A * 10/1997 Granzow...
USOO6920822B2 (12) United States Patent (10) Patent No.: Finan (45) Date of Patent: Jul. 26, 2005 (54) DIGITAL CAN DECORATING APPARATUS 5,186,100 A 2/1993 Turturro et al. 5,677.719 A * 10/1997 Granzow...
10 ROTARY-TUBE PROCESSORS
 10 ROTARY-TUBE PROCESSORS STEPS AND CONDITIONS Table 10-1 Steps and Conditions Rotary-Tube Processors Step Time* (Minutes:Seconds) Temperature C ( F) * All times include a 10- to 20-second drain time.
10 ROTARY-TUBE PROCESSORS STEPS AND CONDITIONS Table 10-1 Steps and Conditions Rotary-Tube Processors Step Time* (Minutes:Seconds) Temperature C ( F) * All times include a 10- to 20-second drain time.
Two Categories of Metal Casting Processes
 Two Categories of Metal Casting Processes 1. Expendable mold processes - mold is sacrificed to remove part Advantage: more complex shapes possible Disadvantage: production rates often limited by time to
Two Categories of Metal Casting Processes 1. Expendable mold processes - mold is sacrificed to remove part Advantage: more complex shapes possible Disadvantage: production rates often limited by time to
raft for comments only Not to be cited as East African Standard FINAL DRAFT EAST AFRICAN STANDARD EAST AFRICAN COMMUNITY
 ICS 71.100 HS 3401.19.00 FINAL DRAFT EAST AFRICAN STANDARD Laundry soap Specification EAST AFRICAN COMMUNITY EAS 2011 Second Edition 2011 ii Table of contents Introduction... iv 1 Scope... 1 2 Definitions...
ICS 71.100 HS 3401.19.00 FINAL DRAFT EAST AFRICAN STANDARD Laundry soap Specification EAST AFRICAN COMMUNITY EAS 2011 Second Edition 2011 ii Table of contents Introduction... iv 1 Scope... 1 2 Definitions...
Paper Ink Preparation by Three Roll Mill
 Paper Ink Preparation by Three Roll Mill 1. INTRODUCTION Printing of one form or another has been with us for centuries and whilst the technologies of both the printing process and the ink formulations
Paper Ink Preparation by Three Roll Mill 1. INTRODUCTION Printing of one form or another has been with us for centuries and whilst the technologies of both the printing process and the ink formulations
Special Casting Process. 1. Permanent mould casting
 Special Casting Process 1. Permanent mould casting A permanent mold casting makes use of a mold or metallic die which is permanent.molten metal is poured into the mold under gravity only and no external
Special Casting Process 1. Permanent mould casting A permanent mold casting makes use of a mold or metallic die which is permanent.molten metal is poured into the mold under gravity only and no external
United States Patent (19)
 United States Patent (19) Andeweg 54 (76) 22) 21 ) (52) (51) 58 (56) 1955,042 2435,811 2,509,219 2,316,589 INTERNALLY ILLUMINATED CANDLE Inventor: Frits J. Andeweg, 7737 Royal Ln., Dallas, Tex. 75230 Filed:
United States Patent (19) Andeweg 54 (76) 22) 21 ) (52) (51) 58 (56) 1955,042 2435,811 2,509,219 2,316,589 INTERNALLY ILLUMINATED CANDLE Inventor: Frits J. Andeweg, 7737 Royal Ln., Dallas, Tex. 75230 Filed:
United States Patent (19) Cobb
 United States Patent (19) Cobb 54 RAM-SHEAR AND SLIP DEVICE FOR WELL PIPE 75 Inventor: 73) Assignee: A. Tom Cobb, Seabrook, Tex. Continental Oil Company, Ponca City, Okla. 21 Appl. No.: 671,464 22 Filed:
United States Patent (19) Cobb 54 RAM-SHEAR AND SLIP DEVICE FOR WELL PIPE 75 Inventor: 73) Assignee: A. Tom Cobb, Seabrook, Tex. Continental Oil Company, Ponca City, Okla. 21 Appl. No.: 671,464 22 Filed:
The Secret Life of Soap
 The Secret Life of Soap Kevin M. Dunn Spring 2011 $Revision: 1.2 $ 1 Soap Research at Hampen-Sydney College Copyright 2011 Kevin M. Dunn Soap Research Soda Ash and Other White Blemishes Background Chemistry
The Secret Life of Soap Kevin M. Dunn Spring 2011 $Revision: 1.2 $ 1 Soap Research at Hampen-Sydney College Copyright 2011 Kevin M. Dunn Soap Research Soda Ash and Other White Blemishes Background Chemistry
March 6, 1962 W, E, MITCHELL 3,023,968 RECIRCULATING PAINT SPRAY SYSTEM INVENTOR. 2% 4.2% A. $227-2,724. as-1
 March 6, 1962 W, E, MITCHELL RECIRCULATING PAINT SPRAY SYSTEM Filed Sept. 22, 198 2 Sheets-Sheet in INVENTOR. 2% 4.2% A. $227-2,724. as-1 March 6, 1962 W. E. MITCHEL. RECIRCULATING PAINT SPRAY SYSTEM Filed
March 6, 1962 W, E, MITCHELL RECIRCULATING PAINT SPRAY SYSTEM Filed Sept. 22, 198 2 Sheets-Sheet in INVENTOR. 2% 4.2% A. $227-2,724. as-1 March 6, 1962 W. E. MITCHEL. RECIRCULATING PAINT SPRAY SYSTEM Filed
1st LATIN AMERICAN CANDLE MANUFACTURERS FORUM MACHINERY FOR THE PRODUCTION OF CANDLES
 1st LATIN AMERICAN CANDLE MANUFACTURERS FORUM MACHINERY FOR THE PRODUCTION OF CANDLES Riccardo Rota Officine Meccaniche Pontida srl - ITALY 1 3 METHODS FOR THE PRODUCTION OF CANDLES 1. Molten (liquid)
1st LATIN AMERICAN CANDLE MANUFACTURERS FORUM MACHINERY FOR THE PRODUCTION OF CANDLES Riccardo Rota Officine Meccaniche Pontida srl - ITALY 1 3 METHODS FOR THE PRODUCTION OF CANDLES 1. Molten (liquid)
BOOK V CHAPTER 1 BOOK V INTRODUCTION
 BOOK V CHAPTER 1 BOOK V INTRODUCTION 965. Chemists who follow Stahl s 1 approach and believe that the acid obtained when distilling oil is one of the constituent principles of that oil, have in general
BOOK V CHAPTER 1 BOOK V INTRODUCTION 965. Chemists who follow Stahl s 1 approach and believe that the acid obtained when distilling oil is one of the constituent principles of that oil, have in general
United States Patent (19)
 United States Patent (19) Killmeyer (54) APPARATUS FOR MAKING PULTRUDED PRODUCT (75) Inventor: Charles W. Killmeyer, Pittsburgh, Pa. 73) Assignee: PPG Industries, Inc., Pittsburgh, Pa. (21) Appl. No.:
United States Patent (19) Killmeyer (54) APPARATUS FOR MAKING PULTRUDED PRODUCT (75) Inventor: Charles W. Killmeyer, Pittsburgh, Pa. 73) Assignee: PPG Industries, Inc., Pittsburgh, Pa. (21) Appl. No.:
TOILET SOAP STARTER KIT PRODUCTION MANUAL SINGLE SPARK. Your business-in-a-box!
 TOILET SOAP STARTER KIT PRODUCTION MANUAL SINGLE SPARK Your business-in-a-box! CHAPTER ONE EQUIPMENT Here is an overview of the potentially required equipment, including a short description. Soap kettle
TOILET SOAP STARTER KIT PRODUCTION MANUAL SINGLE SPARK Your business-in-a-box! CHAPTER ONE EQUIPMENT Here is an overview of the potentially required equipment, including a short description. Soap kettle
United States Patent (19) Kendziorski
 United States Patent (19) Kendziorski 11) 45) Patent Number: Date of Patent: Dec. 18, 1984 (54) (76) (21) 22 (51) (52) (58) 56 FIREWOOD QUARTERING SPLITTER WEDGE Inventor: John F. Kendiziorski, 49756 York
United States Patent (19) Kendziorski 11) 45) Patent Number: Date of Patent: Dec. 18, 1984 (54) (76) (21) 22 (51) (52) (58) 56 FIREWOOD QUARTERING SPLITTER WEDGE Inventor: John F. Kendiziorski, 49756 York
(( Manufacturing )) Fig. (1): Some casting with large or complicated shape manufactured by sand casting.
 (( Manufacturing )) Expendable Mold Casting Processes: Types of expendable mold casting are: 1 ) Sand casting. 2 ) Shell molding. 3 ) Vacuum molding. 4 ) Investment casting. 5 ) Expanded polystyrene process.
(( Manufacturing )) Expendable Mold Casting Processes: Types of expendable mold casting are: 1 ) Sand casting. 2 ) Shell molding. 3 ) Vacuum molding. 4 ) Investment casting. 5 ) Expanded polystyrene process.
Creams, Ointments, Scrubs & Soaps
 Creams, Ointments, Scrubs & Soaps An ointment is beeswax melted in an oil. You get a lotion, if you add a cup of clean water and blend it slowly together. You need to use an electric blender that the emulsion
Creams, Ointments, Scrubs & Soaps An ointment is beeswax melted in an oil. You get a lotion, if you add a cup of clean water and blend it slowly together. You need to use an electric blender that the emulsion
Manufacture of Cast Products
 Manufacture of Cast Products When a layer of rubber is deposited on the interior surface of a hollow mould, it is known as casting. The latex products obtained by the casting process are hollow and toys,
Manufacture of Cast Products When a layer of rubber is deposited on the interior surface of a hollow mould, it is known as casting. The latex products obtained by the casting process are hollow and toys,
75 Inventors: Onofre Costilla-Vela, Nuevo Leon; : R. SS II.
 USOO5924.47OA United States Patent (19) 11 Patent Number: 5,924,470 Costilla-Vela et al. (45) Date of Patent: Jul. 20, 1999 54 METHOD FOR PREHEATING MOLDS FOR 1-91960 4/1989 Japan... 164/457 ALUMINUM CASTINGS
USOO5924.47OA United States Patent (19) 11 Patent Number: 5,924,470 Costilla-Vela et al. (45) Date of Patent: Jul. 20, 1999 54 METHOD FOR PREHEATING MOLDS FOR 1-91960 4/1989 Japan... 164/457 ALUMINUM CASTINGS
TABLE OF CONTENTS. SI No Contents Page No.
 TABLE OF CONTENTS SI No Contents Page No. 1 Basic Textile Wet Processing Terms 1 2 Sequence of operations in Wet processing 2 3 Brief Note on jigger machine 3 4 Details of jigger machine 4 5 Operating
TABLE OF CONTENTS SI No Contents Page No. 1 Basic Textile Wet Processing Terms 1 2 Sequence of operations in Wet processing 2 3 Brief Note on jigger machine 3 4 Details of jigger machine 4 5 Operating
United States Patent (19)
 USOO6103050A 11 Patent Number: Krueger (45) Date of Patent: Aug. 15, 2000 United States Patent (19) 54 METHOD OF LASER SLITTING AND 5,500,503 3/1996 Pernicka et al.. SEALING TWO FILMS 5,502,292 3/1996
USOO6103050A 11 Patent Number: Krueger (45) Date of Patent: Aug. 15, 2000 United States Patent (19) 54 METHOD OF LASER SLITTING AND 5,500,503 3/1996 Pernicka et al.. SEALING TWO FILMS 5,502,292 3/1996
(12) United States Patent (10) Patent No.: US 6,663,057 B2
 USOO6663057B2 (12) United States Patent (10) Patent No.: US 6,663,057 B2 Garelick et al. (45) Date of Patent: Dec. 16, 2003 (54) ADJUSTABLE PEDESTAL FOR BOAT 5,297.849 A * 3/1994 Chancellor... 297/344.
USOO6663057B2 (12) United States Patent (10) Patent No.: US 6,663,057 B2 Garelick et al. (45) Date of Patent: Dec. 16, 2003 (54) ADJUSTABLE PEDESTAL FOR BOAT 5,297.849 A * 3/1994 Chancellor... 297/344.
Sa Sass. (12) Patent Application Publication (10) Pub. No.: US 2017/ A1. (19) United States. (43) Pub. Date: Apr. 27, PACK et al.
 (19) United States US 201701 12163A1 (12) Patent Application Publication (10) Pub. No.: US 2017/0112163 A1 PACK et al. (43) Pub. Date: Apr. 27, 2017 (54) STAMP PLATE WITH MOULDING STOP (71) Applicant:
(19) United States US 201701 12163A1 (12) Patent Application Publication (10) Pub. No.: US 2017/0112163 A1 PACK et al. (43) Pub. Date: Apr. 27, 2017 (54) STAMP PLATE WITH MOULDING STOP (71) Applicant:
(12) United States Patent (10) Patent No.: US 6,290,055 B1
 USOO62900.55B1 (12) United States Patent (10) Patent No.: Glorfield (45) Date of Patent: Sep. 18, 2001 (54) DEVICE FOR ORIENTING AND ACHIEVING THE OPTIMAL DENSITY OF A QUANTITY 4,732,066 * 3/1988 Del Fabro
USOO62900.55B1 (12) United States Patent (10) Patent No.: Glorfield (45) Date of Patent: Sep. 18, 2001 (54) DEVICE FOR ORIENTING AND ACHIEVING THE OPTIMAL DENSITY OF A QUANTITY 4,732,066 * 3/1988 Del Fabro
OSP Interactive Educational Programming
 OSP Interactive Educational Programming Lesson Title: Pioneer Life in the Okefenokee Swamp (History & Science Connections) OSP Educational Programming: Enhance our focus on Native American and early Okefenokee
OSP Interactive Educational Programming Lesson Title: Pioneer Life in the Okefenokee Swamp (History & Science Connections) OSP Educational Programming: Enhance our focus on Native American and early Okefenokee
Scientific Soapmaking
 Scientific Soapmaking Kevin M. Dunn Summer 2008 $Revision: 1.1 $ 1 Acknowledgements Copyright 2008 Kevin M. Dunn Acknowledgements Mike Lawson/Columbus Foods 2 Why Teach Soapmaking? Why Teach Soapmaking?
Scientific Soapmaking Kevin M. Dunn Summer 2008 $Revision: 1.1 $ 1 Acknowledgements Copyright 2008 Kevin M. Dunn Acknowledgements Mike Lawson/Columbus Foods 2 Why Teach Soapmaking? Why Teach Soapmaking?
Student Laboratory Investigation The Chemistry of Combustion and Respiration. Investigation Procedure:
 Student Laboratory Investigation The Chemistry of Combustion and Respiration Objective On a quiz that follows you will be asked to: 1. Demonstrate how a chemical equation is written 2. Write the equation
Student Laboratory Investigation The Chemistry of Combustion and Respiration Objective On a quiz that follows you will be asked to: 1. Demonstrate how a chemical equation is written 2. Write the equation
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0062354 A1 Ward US 2003.0062354A1 (43) Pub. Date: (54) (76) (21) (22) (60) (51) (52) WIRE FEED SPEED ADJUSTABLE WELDING TORCH
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0062354 A1 Ward US 2003.0062354A1 (43) Pub. Date: (54) (76) (21) (22) (60) (51) (52) WIRE FEED SPEED ADJUSTABLE WELDING TORCH
United States Patent [15] 3,650,496 Svensson (45) Mar. 21, 1972
![United States Patent [15] 3,650,496 Svensson (45) Mar. 21, 1972 United States Patent [15] 3,650,496 Svensson (45) Mar. 21, 1972](/thumbs/91/105347464.jpg) United States Patent [15] 3,650,496 Svensson (45) Mar. 21, 1972 54. FOLDING FNS FOR MESSELES 3,273,500 9/1966 Kongelbeck... 244/3.28 (72) Inventor: Nils-Åke Birger Svensson, Karlskoga, Primary Examiner-Verlin
United States Patent [15] 3,650,496 Svensson (45) Mar. 21, 1972 54. FOLDING FNS FOR MESSELES 3,273,500 9/1966 Kongelbeck... 244/3.28 (72) Inventor: Nils-Åke Birger Svensson, Karlskoga, Primary Examiner-Verlin
4.1.3: Shell Casting.
 4.1.3: Shell Casting. It is another expandable mold casting type; Shell molding is a casting process in which the mold is a thin shell (typically 9mm) made of sand held together by a thermosetting resin
4.1.3: Shell Casting. It is another expandable mold casting type; Shell molding is a casting process in which the mold is a thin shell (typically 9mm) made of sand held together by a thermosetting resin
April 1, 1969 W. JONAs ET AL 3,435,988. PAPER Cup DISPENSER. Filed March 20, 1968 Sheet / of 2 N S. INVENTORs WALTER JONAS. ADOLF PFUND. ATTORNEY.
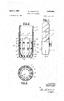 April 1, 1969 W. JONAs ET AL. PAPER Cup DISPENSER Filed March 20, 1968 Sheet / of 2 N S. N ) INVENTORs WALTER JONAS. ADOLF PFUND. ATTORNEY. April 1, 1969 filed March 20, 1968 Sºzzzzzzzz!,, ~~~~ FIG 5.
April 1, 1969 W. JONAs ET AL. PAPER Cup DISPENSER Filed March 20, 1968 Sheet / of 2 N S. N ) INVENTORs WALTER JONAS. ADOLF PFUND. ATTORNEY. April 1, 1969 filed March 20, 1968 Sºzzzzzzzz!,, ~~~~ FIG 5.
Drawing. Fig. 1 Drawing
 Drawing Drawing is a metalworking process which uses tensile forces to stretch metal. It is broken up into two types: sheet metal drawing and wire, bar, and tube drawing. The specific definition for sheet
Drawing Drawing is a metalworking process which uses tensile forces to stretch metal. It is broken up into two types: sheet metal drawing and wire, bar, and tube drawing. The specific definition for sheet
International Journal of Engineering & Technology IJET-IJENS Vol: 12 No: 01 5
 International Journal of Engineering & Technology IJET-IJENS Vol: 12 No: 01 5 Advantages of Prewashed 100 % cotton knit fabric over Scoured Bleached fabric in deep color Reactive dyeing process. Asma Begum
International Journal of Engineering & Technology IJET-IJENS Vol: 12 No: 01 5 Advantages of Prewashed 100 % cotton knit fabric over Scoured Bleached fabric in deep color Reactive dyeing process. Asma Begum
Appl. No.: 619,775 Filed: Nov. 29, 1990 Int. Cl... E21B 4/02 U.S. Cl /907. 1; 175/ /95, 97, 282,303,
 United States Patent (19) Justman et al. (54) (75) (73) 21 22 (51) (52) (58) 56) BEARING STRUCTURE FOR DOWNHOLE MOTORS Inventors: Dan B. Justman, Houston; George A. Cross, Kingwood, both of Tex. Assignee:
United States Patent (19) Justman et al. (54) (75) (73) 21 22 (51) (52) (58) 56) BEARING STRUCTURE FOR DOWNHOLE MOTORS Inventors: Dan B. Justman, Houston; George A. Cross, Kingwood, both of Tex. Assignee:
United States Patent [19]
![United States Patent [19] United States Patent [19]](/thumbs/90/101999790.jpg) United States Patent [19] Landeis 111111 1111111111111111111111111111111111111111111111111111111111111 US005904033A [11] Patent Number: [45] Date of Patent: May 18, 1999 [54] VINE CUTTER [76] Inventor:
United States Patent [19] Landeis 111111 1111111111111111111111111111111111111111111111111111111111111 US005904033A [11] Patent Number: [45] Date of Patent: May 18, 1999 [54] VINE CUTTER [76] Inventor:
United States Patent (19) Fries
 4, 297 0 () () United States Patent (19) Fries 4). SOLAR LIGHTING SYSTEM 76) Inventor: James E. Fries, 7860 Valley View, Apt. 242, Buena Park, Calif. 90620 (21) Appl. No.: 2,620 22 Filed: Jan. 11, 1979
4, 297 0 () () United States Patent (19) Fries 4). SOLAR LIGHTING SYSTEM 76) Inventor: James E. Fries, 7860 Valley View, Apt. 242, Buena Park, Calif. 90620 (21) Appl. No.: 2,620 22 Filed: Jan. 11, 1979
Paper and Pulp Industry
 Paper and Pulp Industry What is a Pulp? Pulp is a lignocellulosic fibrous material Prepared by chemically or mechanically separating cellulose fibres from wood, fibre crops or waste paper. The wood fiber
Paper and Pulp Industry What is a Pulp? Pulp is a lignocellulosic fibrous material Prepared by chemically or mechanically separating cellulose fibres from wood, fibre crops or waste paper. The wood fiber
Subject : Dyeing And Printing. Unit 5: Dyeing process for natural fibers. Quadrant 1 E-Text
 Subject : Dyeing And Printing Unit 5: Dyeing process for natural fibers Quadrant 1 E-Text Learning Objectives The learning objectives of this unit are: Describe the dyeing process for cellulosic fibers
Subject : Dyeing And Printing Unit 5: Dyeing process for natural fibers Quadrant 1 E-Text Learning Objectives The learning objectives of this unit are: Describe the dyeing process for cellulosic fibers
Uniperol EL. Technical Information. Nonionic dispersing agent, emulsifier and leveling agent for use in textile dyeing and printing processes.
 Technical Information Uniperol EL September 1999 Nonionic dispersing agent, emulsifier and leveling agent for use in textile dyeing and printing processes. Colorants and Finishing Products Nature Ethoxylation
Technical Information Uniperol EL September 1999 Nonionic dispersing agent, emulsifier and leveling agent for use in textile dyeing and printing processes. Colorants and Finishing Products Nature Ethoxylation
United States Patent (19) Green et al.
 United States Patent (19) Green et al. (54. FOLDABLE BINOCULARS 76 Inventors: John R. Green, 3105 E. Harcourt St., Compton, Calif. 90221; Charles D. Turner, 48 Eastfield Dr., Rolling Hills, Calif. 90274
United States Patent (19) Green et al. (54. FOLDABLE BINOCULARS 76 Inventors: John R. Green, 3105 E. Harcourt St., Compton, Calif. 90221; Charles D. Turner, 48 Eastfield Dr., Rolling Hills, Calif. 90274
(12) United States Patent (10) Patent No.: US 6,386,952 B1
 USOO6386952B1 (12) United States Patent (10) Patent No.: US 6,386,952 B1 White (45) Date of Patent: May 14, 2002 (54) SINGLE STATION BLADE SHARPENING 2,692.457 A 10/1954 Bindszus METHOD AND APPARATUS 2,709,874
USOO6386952B1 (12) United States Patent (10) Patent No.: US 6,386,952 B1 White (45) Date of Patent: May 14, 2002 (54) SINGLE STATION BLADE SHARPENING 2,692.457 A 10/1954 Bindszus METHOD AND APPARATUS 2,709,874
Oxygen Release Compound (ORC ) Installation Instructions (Excavation Applications)
 Oxygen Release Compound (ORC ) Installation Instructions (Excavation Applications) SAFETY: Pure ORC is shipped to you as a fine powder, which is rated at -325 mesh (passes through a 44 micron screen).
Oxygen Release Compound (ORC ) Installation Instructions (Excavation Applications) SAFETY: Pure ORC is shipped to you as a fine powder, which is rated at -325 mesh (passes through a 44 micron screen).
Optimizing feeding accuracy for your batch or continuous process
 As appeared in December 2015 PBE www.powderbulk.com Optimizing feeding accuracy for your batch or continuous process Sharon Nowak Coperion K-Tron For many bulk solids manufacturing operations, feeding
As appeared in December 2015 PBE www.powderbulk.com Optimizing feeding accuracy for your batch or continuous process Sharon Nowak Coperion K-Tron For many bulk solids manufacturing operations, feeding
11) Patent Number: 5,323,091 Morris (45) Date of Patent: Jun. 21, STARTING SOURCE FOR ARC DISCHARGE 4,041,352 8/1977 McNeill et al...
 IIIHIIII USOO5323091A United States Patent (19) 11) Patent Number: 5,323,091 Morris (45) Date of Patent: Jun. 21, 1994 54 STARTING SOURCE FOR ARC DISCHARGE 4,041,352 8/1977 McNeill et al.... 315/248 LAMPS
IIIHIIII USOO5323091A United States Patent (19) 11) Patent Number: 5,323,091 Morris (45) Date of Patent: Jun. 21, 1994 54 STARTING SOURCE FOR ARC DISCHARGE 4,041,352 8/1977 McNeill et al.... 315/248 LAMPS
Model 511 Model 711 Model 821
 Stainless Steel Tru-Balance Model 511 Model 711 Model 821 Shown with front lift off shield removed The ultimate in reliable performance, capacity, versatility and sanitation for your sifting or screening
Stainless Steel Tru-Balance Model 511 Model 711 Model 821 Shown with front lift off shield removed The ultimate in reliable performance, capacity, versatility and sanitation for your sifting or screening
(12) United States Patent (10) Patent No.: US 6,681,489 B1. Fleming (45) Date of Patent: Jan. 27, 2004
 USOO6681489B1 (12) United States Patent (10) Patent No.: Fleming (45) Date of Patent: Jan. 27, 2004 (54) METHOD FOR MANUFACTURING A 5,732,582 A 3/1998 Knudson... 72/131 VEHICLE FRAME ASSEMBLY 5,855,394
USOO6681489B1 (12) United States Patent (10) Patent No.: Fleming (45) Date of Patent: Jan. 27, 2004 (54) METHOD FOR MANUFACTURING A 5,732,582 A 3/1998 Knudson... 72/131 VEHICLE FRAME ASSEMBLY 5,855,394
Metal Casting Processes CHAPTER 11 PART I
 Metal Casting Processes CHAPTER 11 PART I Topics Introduction Sand casting Shell-Mold Casting Expendable Pattern Casting Plaster-Mold Casting Introduction Metal-Casting Processes First casting were made
Metal Casting Processes CHAPTER 11 PART I Topics Introduction Sand casting Shell-Mold Casting Expendable Pattern Casting Plaster-Mold Casting Introduction Metal-Casting Processes First casting were made
Chapter 1 Sand Casting Processes
 Chapter 1 Sand Casting Processes Sand casting is a mold based net shape manufacturing process in which metal parts are molded by pouring molten metal into a cavity. The mold cavity is created by withdrawing
Chapter 1 Sand Casting Processes Sand casting is a mold based net shape manufacturing process in which metal parts are molded by pouring molten metal into a cavity. The mold cavity is created by withdrawing
United States Patent (19) Ortloff et al.
 United States Patent (19) Ortloff et al. 54) (75) THREADED PIPE CONNECTION HAVING WEDGE THREADS Inventors: Donald J. Ortloff; Doyle E. Reeves, both of Houston, Tex. 73 Assignee: Hydril Company, Houston,
United States Patent (19) Ortloff et al. 54) (75) THREADED PIPE CONNECTION HAVING WEDGE THREADS Inventors: Donald J. Ortloff; Doyle E. Reeves, both of Houston, Tex. 73 Assignee: Hydril Company, Houston,
United States Patent (19) Morita et al.
 United States Patent (19) Morita et al. - - - - - 54. TEMPLATE 75 Inventors: Shiro Morita, Sakura; Kazuo Yoshitake, Tokyo, both of Japan 73 Assignee: Yoshitake Seisakujo Co., Inc., Tokyo, Japan (21) Appl.
United States Patent (19) Morita et al. - - - - - 54. TEMPLATE 75 Inventors: Shiro Morita, Sakura; Kazuo Yoshitake, Tokyo, both of Japan 73 Assignee: Yoshitake Seisakujo Co., Inc., Tokyo, Japan (21) Appl.
United States Patent (19) Schoonover et al.
 United States Patent (19) Schoonover et al. (54) 76 (21) 22 (51) (52) (58) 56) FLUID CONTAINER Inventors: Michael I. Schoonover, 1218 W. Atherton, Flint, Mich. 48507; James A. McFadden, 504 Kingswood,
United States Patent (19) Schoonover et al. (54) 76 (21) 22 (51) (52) (58) 56) FLUID CONTAINER Inventors: Michael I. Schoonover, 1218 W. Atherton, Flint, Mich. 48507; James A. McFadden, 504 Kingswood,
(12) United States Patent (10) Patent No.: US 9,068,465 B2
 USOO90684-65B2 (12) United States Patent (10) Patent No.: Keny et al. (45) Date of Patent: Jun. 30, 2015 (54) TURBINE ASSEMBLY USPC... 416/215, 216, 217, 218, 248, 500 See application file for complete
USOO90684-65B2 (12) United States Patent (10) Patent No.: Keny et al. (45) Date of Patent: Jun. 30, 2015 (54) TURBINE ASSEMBLY USPC... 416/215, 216, 217, 218, 248, 500 See application file for complete
United States Patent (19)
 United States Patent (19) Mongoven et al. (54) 75 73) 21 22 (51) (52) 58) 56 POWER CRCUT FOR SERIES CONNECTED LOADS Inventors: Michael A. Mongoven, Oak Park; James P. McGee, Chicago, both of 1. Assignee:
United States Patent (19) Mongoven et al. (54) 75 73) 21 22 (51) (52) 58) 56 POWER CRCUT FOR SERIES CONNECTED LOADS Inventors: Michael A. Mongoven, Oak Park; James P. McGee, Chicago, both of 1. Assignee:
WOOD 474 Structural Panels. Plywood
 WOOD 474 Structural Panels Plywood 1 Oriented Strand Board Structural panels Plywood Produced from veneers glued together at right angles to maximise stability and strength. Oriented Strand Board (OSB)
WOOD 474 Structural Panels Plywood 1 Oriented Strand Board Structural panels Plywood Produced from veneers glued together at right angles to maximise stability and strength. Oriented Strand Board (OSB)
Fig. 3. BY r: 42.e4.14ce. Oct. 13, 1970 H. HEITMULLER E.T A. 3,533,197 PLIERS, PARTICULARLY NIPPERS INVENTOR.
 Oct. 13, 1970 H. HEITMULLER E.T A. 3,533,197 METHOD OF SHARPENING THE CUTTING EDGES OF SIDE CUTTING Filed March 27, 1967 PLIERS, PARTICULARLY NIPPERS 4. Sheets-Sheet Fig. 3 4 BY r: INVENTOR. 42.e4.14ce
Oct. 13, 1970 H. HEITMULLER E.T A. 3,533,197 METHOD OF SHARPENING THE CUTTING EDGES OF SIDE CUTTING Filed March 27, 1967 PLIERS, PARTICULARLY NIPPERS 4. Sheets-Sheet Fig. 3 4 BY r: INVENTOR. 42.e4.14ce
Of the three grades, grade 1 tallow was chosen as the starting material as it appears the most homogenous and the lightest in colour.
 Fuel supplement Studies (Tallow) The limited quantity of fossil fuels has been the primary driving force for research into alternative sources of fuel. Natural materials have always provided Man with combustible
Fuel supplement Studies (Tallow) The limited quantity of fossil fuels has been the primary driving force for research into alternative sources of fuel. Natural materials have always provided Man with combustible
(12) United States Patent (10) Patent No.: US 8,187,032 B1
 US008187032B1 (12) United States Patent (10) Patent No.: US 8,187,032 B1 Park et al. (45) Date of Patent: May 29, 2012 (54) GUIDED MISSILE/LAUNCHER TEST SET (58) Field of Classification Search... 439/76.1.
US008187032B1 (12) United States Patent (10) Patent No.: US 8,187,032 B1 Park et al. (45) Date of Patent: May 29, 2012 (54) GUIDED MISSILE/LAUNCHER TEST SET (58) Field of Classification Search... 439/76.1.
HOW CAN YOU CREATE A GIANT BUBBLE?
 ACTIVITY 3 HOW CAN YOU CREATE A GIANT BUBBLE? EXPERIMENT OBJECTIVES AND CONTENT The goal of this activity is to teach students about soap bubbles. ESSENTIAL KNOWLEDGE Matter Changes in matter: physical
ACTIVITY 3 HOW CAN YOU CREATE A GIANT BUBBLE? EXPERIMENT OBJECTIVES AND CONTENT The goal of this activity is to teach students about soap bubbles. ESSENTIAL KNOWLEDGE Matter Changes in matter: physical
Designation of Soap Molder Machine and Procedure for Transparent Soap
 IOP Conference Series: Materials Science and Engineering PAPER OPEN ACCESS Designation of Soap Molder Machine and Procedure for Transparent Soap To cite this article: Zainon Binti Mat Sharif et al 2017
IOP Conference Series: Materials Science and Engineering PAPER OPEN ACCESS Designation of Soap Molder Machine and Procedure for Transparent Soap To cite this article: Zainon Binti Mat Sharif et al 2017
United States Patent (19)
 United States Patent (19) Raphael et al. USO05433448A 11 Patent Number: Date of Patent: Jul.18, 1995 (54) 76 21 22) (51) (52) (58 THREE-DIMENSIONAL TIC-TAC-TOE GAME Inventors: Stewart C. Raphael; Audrey
United States Patent (19) Raphael et al. USO05433448A 11 Patent Number: Date of Patent: Jul.18, 1995 (54) 76 21 22) (51) (52) (58 THREE-DIMENSIONAL TIC-TAC-TOE GAME Inventors: Stewart C. Raphael; Audrey
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 (19) United States US 2016.0325383A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0325383 A1 Xu et al. (43) Pub. Date: (54) ELECTRON BEAM MELTING AND LASER B23K I5/00 (2006.01) MILLING COMPOSITE
(19) United States US 2016.0325383A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0325383 A1 Xu et al. (43) Pub. Date: (54) ELECTRON BEAM MELTING AND LASER B23K I5/00 (2006.01) MILLING COMPOSITE
How to make soap at home school year prof. Luigi Cenerelli translation prof. Paolo Badiali
 How to make soap at home school year 2014-2015 prof. Luigi Cenerelli translation prof. Paolo Badiali Brief sketch of the history of soap Although the ingredients used to make soap have changed over the
How to make soap at home school year 2014-2015 prof. Luigi Cenerelli translation prof. Paolo Badiali Brief sketch of the history of soap Although the ingredients used to make soap have changed over the
(12) Patent Application Publication (10) Pub. No.: US 2005/ A1
 (19) United States US 2005OO17592A1 (12) Patent Application Publication (10) Pub. No.: Fukushima (43) Pub. Date: Jan. 27, 2005 (54) ROTARY ELECTRIC MACHINE HAVING ARMATURE WINDING CONNECTED IN DELTA-STAR
(19) United States US 2005OO17592A1 (12) Patent Application Publication (10) Pub. No.: Fukushima (43) Pub. Date: Jan. 27, 2005 (54) ROTARY ELECTRIC MACHINE HAVING ARMATURE WINDING CONNECTED IN DELTA-STAR
- I 12 \ C LC2 N28. United States Patent (19) Swanson et al. EMITTERS (22) 11 Patent Number: 5,008,594 (45) Date of Patent: Apr.
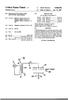 United States Patent (19) Swanson et al. 11 Patent Number: () Date of Patent: Apr. 16, 1991 54 (75) (73) (21) (22) (51) (52) (58) SELF-BALANCNG CIRCUT FOR CONVECTION AIR ONZERS Inventors: Assignee: Appl.
United States Patent (19) Swanson et al. 11 Patent Number: () Date of Patent: Apr. 16, 1991 54 (75) (73) (21) (22) (51) (52) (58) SELF-BALANCNG CIRCUT FOR CONVECTION AIR ONZERS Inventors: Assignee: Appl.
Effect of Slaking Water Temperature on Quality of Hydrated Lime Slurry By: Mohamad Hassibi Chemco Systems, L.P. July, 2009 Revised: June 20, 2015
 Effect of Slaking Water Temperature on Quality of Hydrated Lime Slurry By: Mohamad Hassibi Chemco Systems, L.P. July, 2009 Revised: June 20, 2015 There is a recent debate regarding the impact heated slaking
Effect of Slaking Water Temperature on Quality of Hydrated Lime Slurry By: Mohamad Hassibi Chemco Systems, L.P. July, 2009 Revised: June 20, 2015 There is a recent debate regarding the impact heated slaking
(51) Int. Cl."... Hosk 720 Amachine device that forces filtered air into and through a
 USOO5888134A United States Patent (19) 11 Patent Number: 5,888,134 McNair, Jr. (45) Date of Patent: Mar. 30, 1999 54 EXTERNAL TO INTERNAL LAPTOP 5,725,622 3/1998 Whitson et al.... 454/184 X COMPUTER AND
USOO5888134A United States Patent (19) 11 Patent Number: 5,888,134 McNair, Jr. (45) Date of Patent: Mar. 30, 1999 54 EXTERNAL TO INTERNAL LAPTOP 5,725,622 3/1998 Whitson et al.... 454/184 X COMPUTER AND
52 U.S. Cl... 87/9; 87/56; 228/19. (56) References Cited U.S. PATENT DOCUMENTS Re. 32,086 2/1986 Spirig /19
 United States Patent (19) Forsha (54) DESOLDERING BRAID (75) Inventor: Alan L. Forsha, Upland, Calif. 73) Assignee: Solder Removal Company, Covina, Calif. (21) Appl. No.: 485,104 22 Filed: Feb. 26, 1990
United States Patent (19) Forsha (54) DESOLDERING BRAID (75) Inventor: Alan L. Forsha, Upland, Calif. 73) Assignee: Solder Removal Company, Covina, Calif. (21) Appl. No.: 485,104 22 Filed: Feb. 26, 1990
United States Patent (19) Manfroni
 United States Patent (19) Manfroni 54 scraper AND MIXER ELEMENT FOR ICE CREAM MAKING MACHINES 75) Inventor: Ezio Manfroni, Sasso Marconi, Italy 73 Assignee: Carpigiani Bruto Macchine Automatiche S.P.A.,
United States Patent (19) Manfroni 54 scraper AND MIXER ELEMENT FOR ICE CREAM MAKING MACHINES 75) Inventor: Ezio Manfroni, Sasso Marconi, Italy 73 Assignee: Carpigiani Bruto Macchine Automatiche S.P.A.,
ANIMAL OILS AND FATS CHAPTER 11 CETINE 1 1. COMPOSITION
 132 CHAPTER 11 CETINE 1 1. COMPOSITION 510. BY WEIGHT BY VOLUME 2 Oxygen 5.478 100 1.00 Carbon. 81.660 1490.7 19.48 Hydrogen. 12.862 234.8 37.70 2. PHYSICAL PROPERTIES 511. It melts at 49 C. On cooling
132 CHAPTER 11 CETINE 1 1. COMPOSITION 510. BY WEIGHT BY VOLUME 2 Oxygen 5.478 100 1.00 Carbon. 81.660 1490.7 19.48 Hydrogen. 12.862 234.8 37.70 2. PHYSICAL PROPERTIES 511. It melts at 49 C. On cooling
Troubleshooting Conventional Burnout Phosphate Bonded Investments
 Troubleshooting Conventional Burnout Phosphate Bonded Investments Phosphate investments are affected by many variables, but the following generalizations can be made: Thorough mixing insures complete reaction
Troubleshooting Conventional Burnout Phosphate Bonded Investments Phosphate investments are affected by many variables, but the following generalizations can be made: Thorough mixing insures complete reaction
United States Patent Office
 United States Patent Office PROCESS FOR ANHYDROUS CALCUM 12-HY DROXY STEARATE AND ESTOLIDE CONTAIN NG GREASE John P. Dilworth, Fishkill, N.Y., Charles H. Culmane, Jr., Wyandotte, Mich., and Roy F. Nelson,
United States Patent Office PROCESS FOR ANHYDROUS CALCUM 12-HY DROXY STEARATE AND ESTOLIDE CONTAIN NG GREASE John P. Dilworth, Fishkill, N.Y., Charles H. Culmane, Jr., Wyandotte, Mich., and Roy F. Nelson,
United States Patent (19)
 United States Patent (19) Crawford 11 Patent Number: 45) Date of Patent: Jul. 3, 1990 54 (76) (21) 22 (51) (52) (58) 56 LASERRANGEFINDER RECEIVER. PREAMPLETER Inventor: Ian D. Crawford, 1805 Meadowbend
United States Patent (19) Crawford 11 Patent Number: 45) Date of Patent: Jul. 3, 1990 54 (76) (21) 22 (51) (52) (58) 56 LASERRANGEFINDER RECEIVER. PREAMPLETER Inventor: Ian D. Crawford, 1805 Meadowbend
(12) United States Patent (10) Patent No.: US 6,705,355 B1
 USOO670.5355B1 (12) United States Patent (10) Patent No.: US 6,705,355 B1 Wiesenfeld (45) Date of Patent: Mar. 16, 2004 (54) WIRE STRAIGHTENING AND CUT-OFF (56) References Cited MACHINE AND PROCESS NEAN
USOO670.5355B1 (12) United States Patent (10) Patent No.: US 6,705,355 B1 Wiesenfeld (45) Date of Patent: Mar. 16, 2004 (54) WIRE STRAIGHTENING AND CUT-OFF (56) References Cited MACHINE AND PROCESS NEAN
United States Patent to 11 3,998,002
 United States Patent to 11 Nathanson 45 Dec. 21, 1976 54 PANEL, HOLDER FOR SMALL STRUCTURES AND TOYS 76 Inventor: Albert Nathanson, 249-26 63rd Ave., Little Neck, N.Y. 11329 22 Filed: Jan. 29, 1975 (21
United States Patent to 11 Nathanson 45 Dec. 21, 1976 54 PANEL, HOLDER FOR SMALL STRUCTURES AND TOYS 76 Inventor: Albert Nathanson, 249-26 63rd Ave., Little Neck, N.Y. 11329 22 Filed: Jan. 29, 1975 (21
Schaeff, LLP. 22 Filed: Nov. 2, 1998 (51) Int. Cl."... B21D 51/ U.S. Cl... 72/329; 72/ Field of Search... 72/327, 328, 329, 72/348
 United States Patent Turner et al. 19 USOO607.9249A 11 Patent Number: (45) Date of Patent: Jun. 27, 2000 54 METHODS AND APPARATUS FOR FORMING A BEADED CAN END 75 Inventors: Stephen B. Turner, Kettering;
United States Patent Turner et al. 19 USOO607.9249A 11 Patent Number: (45) Date of Patent: Jun. 27, 2000 54 METHODS AND APPARATUS FOR FORMING A BEADED CAN END 75 Inventors: Stephen B. Turner, Kettering;
United States Patent 19) 11 Patent Number: 5,442,436 Lawson (45) Date of Patent: Aug. 15, 1995
 I () US005442436A United States Patent 19) 11 Patent Number: Lawson (45) Date of Patent: Aug. 15, 1995 54 REFLECTIVE COLLIMATOR 4,109,304 8/1978 Khvalovsky et al.... 362/259 4,196,461 4/1980 Geary......
I () US005442436A United States Patent 19) 11 Patent Number: Lawson (45) Date of Patent: Aug. 15, 1995 54 REFLECTIVE COLLIMATOR 4,109,304 8/1978 Khvalovsky et al.... 362/259 4,196,461 4/1980 Geary......
Marbling Please read through the directions before starting.
 Marbling Please read through the directions before starting. For cotton, silk or any fabric that is absorbent including cotton/polyester blends, 100% polyester or nylon. It is possible to marble on any
Marbling Please read through the directions before starting. For cotton, silk or any fabric that is absorbent including cotton/polyester blends, 100% polyester or nylon. It is possible to marble on any
(12) United States Patent (10) Patent No.: US 7,050,541 B2
 US007050541B (1) United States Patent () Patent No.: Bitt (45) Date of Patent: May 3, 006 (54) X-RAY TUBE WITH LIQUID-METAL FLUID (56) References Cited BEARNG U.S. PATENT DOCUMENTS (75) Inventor: Herbert
US007050541B (1) United States Patent () Patent No.: Bitt (45) Date of Patent: May 3, 006 (54) X-RAY TUBE WITH LIQUID-METAL FLUID (56) References Cited BEARNG U.S. PATENT DOCUMENTS (75) Inventor: Herbert
