Acceptance Solid State NMR Test Procedure. for Avance NMR Systems
|
|
|
- William Harrison
- 6 years ago
- Views:
Transcription
1 Acceptance Solid State NMR Test Procedure for Avance NMR Systems Manual P/N B92999 Acceptance Solid State NMR Test Procedures for AVANCE systems Index: 04 Number: B92999 Page: 1 (42)
2 1. Purpose 2. Area of application 3. Referenced documents 4. NMR experiments for probe tests 4.1 Introduction B 0 -Field Adjustment, Referencing Sample Preparation MAS Monitoring 4.2 Setting the magic angle with KBr H π/2 pulse Adamantane 4.4 Direct polarization 13 C on Adamantane / resolution test 4.5 Cross polarization 1 H - 13 C on Adamantane 4.6 Sensitivity 13 C Glycine C π/2 pulse determination (optional experiment) 4.8 Sensitivity 15 N Glycine N π/2 pulse determination (optional experiment) P CPMAS experiment P π/2 pulse determination (optional experiment) 4.12 Double CP experiment 4.13 Double CP experiment tuning stability test 4.14 HFX experiments HF experiment and FH experiment FC CPMAS experiment FC(H) CPMAS experiment 5. Setup 4Phase Modulator 6. Shimming a CP MAS probe Revision Record: Index Date Text Initials 00 02/01/2001 Created JOS 06/11/2001 Revised HCL, JOS 03/27/2002 Edited ASF, JOS 01 04/28/2003 Revised JOS 09/03/2003 Revised JOS 02 02/24/2004 Revised JOS 03 12/22/2004 Revised JOS, BRC 04 11/11/2005 Extended with DCP and HFC experiments JOS Index: 04 Number: B92999 Page: 2 (42)
3 1. Purpose This manual describes the procedures for the NMR tests that are necessary to set up Solid State NMR MAS experiments in order to demonstrate the high performance of Bruker Avance Instruments. It includes basic specification tests such as 13 C resolution (line width at half height) and sensitivity tests, as well as some advice on shimming MAS probes. More advanced experiments and their setup can be found in the help menu of XWIN-NMR (other topics - solids users manual) or using NMR-Guide. All test procedures are subject to change without notice. 2. Area of application This manual is for all Bruker Service personnel engaged in final testing, service and installation of NMR instruments, as well as for customers. The manual is a complement to the Acceptance and Test Procedures for Avance NMR systems, ZUEP0102. The manual complements the solids part of the ATP program and can be used only for systems with the software Release XWIN-NMR 3.1 patch level 11 and higher with AQS electronics. Note: The CP pulse programs and related parameter sets do not work on systems with AQX/AQR electronics. Pulse programs for D*X systems are available in the software release under <Xwinnmrhome>/exp/stan/nmr/lists/pp.dsolids'. Appropriate adjustments to the parameter sets need to be done as well. 3. Referenced documents ZUEP0102 H9321 Z31401 B3072 Bxxx Acceptance test procedures for Avance spectrometers and documents referenced in ZUEP0102 Solids user manual SB MAS Operation Manual SB Wideline Probe Manual HR-MAS Standard Test Procedures Index: 04 Number: B92999 Page: 3 (42)
4 4. NMR experiments for probe tests 4.1 Introduction B 0 -Field Adjustment, Referencing Solid-state NMR probes have no 2 H lock channel. As a result, referencing is done for each nucleus with standard samples. For a general spectrometer setup one basic referencing is needed and one convenient standard sample is used. The built in frequency list - following R.K. Harris IUPAC recommendations - guarantees appropriate cross-referencing. The general spectrometer referencing is done using the most convenient sample and related nucleus. If, for example, one uses the 1 H resonance of water or TMS if available, the liquid sample is centered in a MAS rotor as outlined below. The parameter set ZGSOLIDS is loaded, using rpar ZGSOLIS all in the command line of XWIN-NMR. The nucleus 1 H is selected either in the eda or in the edsp editor. Before starting, make sure that in eda the parameter locnuc is set to off, and that neither the lock nor the sweep buttons on the BSMS keypad are lit. If no keypad is available, open the BSMS display/lock window and check the state of field sweep and lock. Both must be off. The 1 H transmitter offset O1P is set to 4.7 ppm, temperature should be around room temperature, furthermore, a recycling delay d1 = 0.5 seconds, td = 4096 and sw = 20ppm is chosen. For excitation one uses a power level of pl1 = 10 db and a pulse length p1 = 5 us. The interactive experiment is started by typing gs into the command line. In order to observe the signal, switch to the acquisition window by typing acqu. Dial the field (BSMS keyboard or BSMS display / shim window) while pulsing, until the FID is on resonance. Then the experiment is stopped by typing stop and an acquisition is started with ns =1 and ds = 0, by typing zg into the command line. The signal is processed with ft and appropriately phased. When TMS is used in the sample, change the BSMS field until the TMS signal is exactly on Zero the Zero frequency position. Sample Nucleus Shift (ppm) Water 1 H 4.7 TMS 1 H 0 KBr 79 Br About 60 ppm R.K. Harris IUPAC recommendation, updated in TOPSPIN release from XWINNMR 3.1 where it was 41.1ppm Adamantane 13 C (Earl et al. JMR 48, 50 (1982) TTMSS 29 Si -8.9 Glycine 13 C 176 (Carbonyl line) Table 1: Some chemical shifts that one can use for referencing. Index: 04 Number: B92999 Page: 4 (42)
5 The procedure has the advantage of allowing the spectrometer frequencies in the parameter sets to be used more easily on other spectrometers (if these spectrometers are referenced as well and use Bruker's frequency list). With O1 and O2 in ppm, different magnetic fields do not matter for parameter transfer Sample Preparation A water sample should be made with a CRAMPS insert or, if that is not available, with some Teflon tape at the bottom of the rotor to center the sample. A drop of water with or without TMS is set on top of the insert or Teflon tape and the upper part of the rotor remains unfilled. Insert the rotor into the probe and do not spin. Some shimming may be done on this sample to improve the line width, using the x or y and z shims. It is advantageous to prepare one rotor with a mixture of KBr and adamantine (except for the 2.5 mm rotors, for reasons of signal intensity). This sample is easier to pack than a pure KBr sample and a number of setup experiments can be done without changing the sample. The 79 Br resonance of KBr is used to set the magic angle. This process is described in section 4.2. Pulse lengths for 13 C and 1 H and the HH contact are determined on the adamantane 13 C (and 1 H) signals. A second rotor filled with Glycine is needed to determine the 13 C and 15 N sensitivity. A rotor filled with ammonium-dihydrogenphosphate is used to measure 31 P signals, determine pulse lengths and sensitivity ( 31 P has no official sensitivity specification but the experiment is useful for obtaining an overall impression of the probe). For SB CPMAS probes and 2.5 mm probes it is advantageous to have a sample with 15 N labeled glycine or ammonium chloride available to determine pulse lengths and power levels needed for the 15 N cross polarization (CP) experiment MAS monitoring In order to monitor the spinning speed of the rotor, the mascontrol window must be opened. To do this, type mascontrol into the command line. Within this window the present probe needs to be selected, open the button etc and select Write log file ON. This will write spin rates to log file so that spin stability can be determined. A printout of this file must be attached to the other test results. Index: 04 Number: B92999 Page: 5 (42)
6 ppm Spectrum with spinning sidebands at 7 khz sample spinning using 100 khz sweep width, digmod = ANALOG. Insert shows the folding back of spinning sidebands. Index: 04 Number: B92999 Page: 6 (42)
7 4.2 Setting the magic angle with KBr For more information on how to adjust the angle using the FID in the gs mode, see manual Z31401 page 45 ff.. Test Sample: Spinning rate: Spectrometer edsp: Parameter set : Pulse program: KBr or KBr +Adamantane 7 khz NUC1 KBr 79 Br ;zg ;#include <Avance.incl> 1 ze ;pl1=power level f1 channel 2 d1 ;p1 =f1 channel high power pulse p1 ph1 ;d1 =relaxation delay = 1-5* T1 go=2 ph31 wr #0 ;write data to disk exit ph1 = ph31= Parameters: Acquisition: PULPROG zg NS 4 P1 4us DW 5us D1 500ms PL1 as determined in the following: DIGMOD analog O1P 41 ppm AQ about 8 msec MASR 6000 Hz and higher NOT a multiple of 5 khz Setting Transmitter power: If the correct power level for the X-channel is not already known, start using 50% of the maximum power for the used channel using the power handling data sheet that comes with each probe. For the appropriate power level settings use 50% of the nominal transmitter power for the power level 0 if CORTAB has not been done and 25% if it has been done. Then use the fact that the power decreases or increases by 50% for a 3dB change above 0dB and below 0dB only if CORTAB has been done. As an example we want to use 75W on a 2.5 mm CPMAS probe with a linearized (CORTAB done) 1000W transmitter. Then we have 250W at 0 db, 125W at 3 db and 75 W at about 5.5 db. As a reminder db is defined as: Index: 04 Number: B92999 Page: 7 (42)
8 power1 ( ) ydb = 10 log. power 2 That is exactly what you can use to calculate the power level change for a certain change of the pulse width or a nutation frequency, but as you are using amplitudes and not power it changes to: ydb ( ) pulsewidth 1 = 20 log pulsewidth 2. In order to find a new pulse width for a given power level it reads: pulsewidth 2 = pulsewidth 1 10 ( powerlevel 1 powerlevel 2 ) / Read in the parameter set KBr, by typing rpar KBr all into the command line. 2. Set the power level PL1 following the suggestions above; match and tune the probe. 3. Type edasp and check the default button, the routing should now go through the high power amplifiers. 4. Type wobb and toggle with acqu into the acquisition window. 5. Match and tune the probe. 6. Type gs in the command line and switch with acqu to the acquisition window. 7. Make fine adjustment of O1 in the gs-window until the signal is full on resonance and set the receiver phase, phref, so that the full signal is in one channel in the ush (unshuffle) mode. 8. Adjust the magic angle, observing the FID and dialing the MA adjustment so that the spinning sidebands extend as far as possible. The spinning sidebands are seen in the time domain signal, the free induction decay (FID), as spikes riding on the on resonance signal of the central transition. The sidebands should extend as far as possible to the end of the 8-10 ms acquisition time. 9. Stop gs and start zg to get a spectrum. Plotting: Plot the whole spectrum Note: A sweep width of 250 khz can also be used to see the whole spinning sideband pattern. Such a sweep width can be achieved either in analog mode or in digital mode with DSPFIRM on smooth. At a spinning rate of 6 to 8 khz, one can see the sidebands going almost all the way out to the end of the spectral window (depends on S/N! i.e. rotor size) and being reflected. Print the spectrum as proof for good setting of magic angle, use 16 to 64 transients to obtain a good enough intensity of the reflected signal. A sweep width of 250 khz cannot be achieved on systems using SADC digitizers. For such systems use swh = 100 khz and digmod=analog. Index: 04 Number: B92999 Page: 8 (42)
9 Final Test BH Operator JOS PH MASVTN 500 WB BL7 15N-31P/1H Adamantane 1H Spinning speed 7 khz 2e+004 1e+004 0e+000-1e+004-2e+004 Hz Index: 04 Number: B92999 Page: 9 (42)
10 4.3 1 H 90 degree pulse Adamantane The Adamantane sample is used to determine the 1 H 90º pulse. While this is done a check of sample spinning needs to be performed order to check whether the probe spins well and to demonstrate stability of MAS controller. 7mm, 4mm and 2.5mm MAS probes need to run at the limit of 7 khz, 15 khz and 35 khz respectively. A log file of the MAS controller must be created according to the procedures outlined in sections Print this file as proof that stable spinning at the highest spinning speed was achieved. Make sure that the rotor is not too old if going to the fastest rotation speeds. With normal wear and tear I would suggest that for acceptance tests in the field the rotor should not be older than 50 high speed tests. It is advised to maintain a usage log for all rotors, speed, when filled last, trips and special issues (dropped etc.). Indeed, it is annoying to have a rotor crash in the field. Test Sample: Spinning rate Spectrometer edsp: Parameter Set Pulse program zg Adamantane limit of probe NUC1 ZGSOLIDS 1 H Parameters Acquisition: PULPROG zg TD 2048 NS 4 P1 4us DW 5us DE 6us D1 2s PL1 as determined in the following: DIGMOD digital 01P 2.3 ppm Transmitter power: see 4.1 Plotting: Plot the whole spectrum. Choose Hz for x-axis units. Note: Use the experiment to determine the pl1 for a 4 us 90-degree pulse on 1H, the power level for the decoupling pulse (e.g. 2.5 us for 4mm CPMAS probe) and write the obtained value for the high power decoupling pulse into edprosol 1H F1A1 in the field for p90 (see appendix). Having those data available allows a fast setup of the CP as well as the sensitivity experiment. With CORTAB done, write the power level and for a 2, 3 and 4 us 1 H pulse into the prosol table on F1 use either all solvents or create a solvent solids following the instructions on the ICONNMR manual extension for solid state NMR. Index: 04 Number: B92999 Page: 10 (42)
11 Final Test BH Operator JOS PH MASVTN 500 WB BL7 15N-31P/1H Adamantane 13C Resolution in Hz ppm Index: 04 Number: B92999 Page: 11 (42)
12 C Direct Polarization on Adamantane Direct detection of 13 C is done on Adamantane in order to determine the 90-degree 13 C pulse at various pulse lengths and power levels. The experiment can, in principle, be used for shimming, however, more signal is available through the CP experiment (see 4.5). Begin with aq = 100ms and adjust 90-degree pulses at various pulse lengths as deemed necessary. For shimming, aq can be increased up to 500ms if the 1 H decoupling is kept moderately low, see note. Attention: Be extra careful during that experiment: check aq if sw, dw, or td values are changed and make sure it stays below 0.1 s, otherwise the probe could be damaged. Use power levels that provide a 5us 90-degree pulse for a 4mm probe - see note below. Test Sample: Adamantane Spinning rate: 5 khz or higher Spectrometer edsp: NUC1 Parameter Set: Pulse program: NUC2 DirectC13 hpdec.av 13 C 1 H ;hpdec.av ;acquisition on X with hp proton decoupling 1 ze ;set RCU to replace mode 2 d1 do:f2 ;recycle delay (p1 ph1):f1 ;transmitter pulse on F1 with power level pl1 1u cpds2:f2 ;use cpdprg2 cw or tppm at power pl12 go=2 ph31 ;make sure the adc is finished, turn decoupling off 1m do:f2 wr #0 ;save data in current data set exit ph0= 0 ph1= ph31= ;constant phase for acquisition ;simple pulse phase list ;signal routing corresponds to pulse phase list Parameters: Acquisition: PULPROG hpdec.av TD 2048 NS 4 P1 4us DW 50 µs CPDPRG2 tppm15 (or cw at low field instruments) PL1 approach as determined in 4.2 and 4.3 PL12 pl for 5.5us 90degree on 1 H (45 khz B1 field) for 4 mm and 6.3 us (40 khz) for 7 mm probes Index: 04 Number: B92999 Page: 12 (42)
13 P31 O1P O2P ZGOPTNS 2*p3-0.2us as start value optimize! Might require a decent shim already. 32 ppm 2.3 ppm -Dtppm Plotting: Plot main part of spectrum and determine line width with the au program hwcal. Note the line width in title, if the experiment was used for shimming. Note 1: If no previous referencing has been done using the BSMS field or the BSMS display/lock window, set the right hand line to 29.5 ppm. If the spectrometer is referenced properly with the field and spectrometer frequency, use the values given in the parameter set. If the sr command is used to set the reference, use a frequency right between both lines as the O1 for the experiment. Then, measure the absolute frequency of the left line and multiply that frequency by in order to find the right SFO2 for the 1 H decoupling. Note 2: This experiment can also be used for shimming the probe. Then it might be necessary to set aq to higher values, up to 500ms. In that case, we reduce the decoupling power as much as possible but try to stay high enough so that dipolar coupling cannot influence the line width. A maximum of 500 ms for aq can be used with reduced decoupling power. This is at the power level of a 5 us pulse, equivalent to a B 1 field of approximately 50 khz. for 4 mm probes and 40 khz for 7mm probes. Pay attention that in such cases the recovery delay D1 is 10 s. One must not exceed a maximum of 500 ms for aq. Note 3: In the most recent release the au-program au_cp, changes the decoupler power level used for the experiment based on the acquisition time. The au_program uses and sets a hidden pulse programs permitting aq up to 1s by reducing the amplitude of the decoupler rf signal by 25% for 50ms < aq < 200ms and by 50% for 200ms < aq < 1s. The decoupler power in the prosol table must be 80% of the max. value, i.e. 80kHz for 4mm (3.1us), 56 khz for 7mm (4.5us) and 125 khz for 2.5mm (2.1us) CPMAS probes. Decoupling programs: The decoupling program cw: 0.5u pl=pl up:0 fq=cnst21 jump to 1 TPPM decoupling: TPPM (Two Pulse Phase-Modulation) has been invented 1995 by the Griffin group at MIT: A.E. Bennett, C.M. Rienstra, M. Auger, K.V. Lakshmi, and R.G. Griffin; Heteronuclear decoupling in rotating solids, J. Chem. Phys. 103 (16); (1995) Index: 04 Number: B92999 Page: 13 (42)
14 TPPM decoupling surpasses the traditional cw decoupling and is now standard in solid state NMR experiments. TPPM is not needed for the experiment with adamantane. The decoupling programs tppm: (the frequency offset cnst21 can be set to 0 for these setup experiments) tppm15 0.3u fq=cnst21 0.5u pl=pl12 1 p31:0 p31:15 p31:0 p31:15 p31:0 p31:15 p31:0 p31:15 p31:0 p31:15 p31:0 p31:15 p31:0 p31:15 p31:0 p31:15 p31:0 p30:15 jump to 1 tppm20 0.3u fq=cnst21 0.5u pl=pl12 1 p31:0 p31:20 p31:0 p31:20 p31:0 p31:20 p31:0 p31:20 p31:0 p31:20 p31:0 p31:20 p31:0 p31:20 p31:0 p31:20 p31:0 p30:20 jump to 1 Index: 04 Number: B92999 Page: 14 (42)
15 ppm Index: 04 Number: B92999 Page: 15 (42)
16 4.5 1 H - 13 C Cross Polarization on Adamantane NOTE: The following experiment uses the same power level PL12 for the 90º-pulse p3 and the decoupling pulse in order to allow control and direct measure of the nutation 1 frequency of the decoupling field. PL2 drives the power of the fast shape during the contact. The 3.1 us (80 khz) pulse for 4 mm probes are known from the previous experiment ( 1 H on adamantane). Use parameter set CPADAM, edit the pulse program and disable the acquisition time protection feature by commenting out the line #include <praq.prot> with a ;. Save the pulse program under the original name with your initials as extension, e.g. if xyz is your extension cp.av is renamed to cp.av.xyz. Subtract 3 or 6 db from pl12 if it is set for the 80 khz decoupling power level, i.e. if pl12 for 80 khz is 5dB, use 8 db for acquisition time 50ms < aq < 200ms and 11 db if the acquisition time is 200ms < aq < 1s. The decoupler power in the prosol table must be 80% of the max. value, i.e. 80kHz for 4mm (3.1us), 56 khz for 7mm (4.5us) and 125 khz for 2.5mm (2.1us) CPMAS probes. Set pl1 to the same power level achieved for a 5us 90 o 13 C pulse from the previous hpdec experiment and adjust pl2 for best HH match using popt. Test Sample: Spinning rate: Spectrometer edsp: Parameter set: Pulse program: Adamantane 5 khz NUC1 NUC2 CPADAM 13 C 1 H Parameters: Acquisition: PULPROG cp.av or cp90.av TD 1024 P1 0 P15 5ms SWH 10 khz D1 5s PL1 for 4 or 4.5 us pulse as determine in 4.4 PL db PL2 power level of shaped contact pulse if one uses the 1 The spin nutation frequency is the frequency at which the spins rotate around the rf-field; f nut =γ/2π B 1, B1 is the field strength of the rf field Index: 04 Number: B92999 Page: 16 (42)
17 SPNAM0 PL12 P3 ramp and pl1 for 4 us excitation pulse we need then the power level for 4 or 4.5 us 1H as determined in 4.3 minus 3 db ramp.100 see text goes with pl12 if pl12 was changed by 3 db, multiply the original pulse with with 1.4, if it was changed by 6 db double the original pulse width. If e.g. original pulse parameters were 3.1us at 5 db, change to 8 db gives 3.1*1.4=4.3us, and a change to 11 db gives a 6.2 us pulse width. CPDPRG P31 ZGOPTNS tppm15 (or cw for low field instruments) 2*p3-0.2us (see above) -Dlacq (switches protection file off for long acquisition times see note below for decoupling power limitations at long acquisition times) Note: This experiment is used for shimming the probe and demonstration of power handling capability. It might be necessary to set aq to higher values. In that case, make sure that the decoupling pulse does not exceed 50 khz (for 4 mm probes and 40 khz for 7mm probes) and that the recovery delay D1 is 10 s. Save parameter setas CPADAM. Do not exceed a maximum of 500 ms for aq. Attention: If aq >50ms, then the probe protection increases the attenuation (the power level) by 3dB for 50ms < aq < 200ms and by 6dB for 200ms < aq < 1s in recent pulse programs Index: 04 Number: B92999 Page: 17 (42)
18 Carbonyl peak Aliphatic peak ppm Index: 04 Number: B92999 Page: 18 (42)
19 C Sensitivity on Glycine Having the best HH match from the previous experiment, it is necessary to optimize the HH match on for the glycine sample again if the spinning speed was changed. Spin at N khz where N is defined by the 1H spectrometer frequency divided by 100MHz (e.g. for a MHz spectrometer N=5). Then p3 is measured in a nutation experiment (increment p3 using popt) in order to verify the measurements at the 1 H experiment. Then calculate the power level for a 2.5 us pulse, which is a 100 khz B 1 field (4mm probe) or 75 khz (7mm probe), using pulse in the 1 H adamantane experiment (section 4.3.) Remember, using the au program pulse requires values be placed in pl1 and p1, not pl2 and p3. Set to the obtained new values for p3 and pl12 and repeat the measurement. The obtained pl value in db is then used for pl12. Measure p3 with popt in order to verify the calculation. A good p31, the pulse for TPPM decoupling, is estimated from p3 using p31=2*p3-0.2us. The power handling test should be done at that stage of the test procedure. The experiment must be done with the pulse program cp90.av (cp4c.98 for D*X spectrometers) with the protection features implemented. NOTE: In more recent releases, the pulse program uses the relations file solids_cp. If this is not the case, the use of the relations file can be obtained by adding the line prosol relations=<solids_cp> between the comments and the first line with an include statement into the pulse program. This requires that the power levels and pulses are entered correctly into the prosol table (see appendix). Test Sample: Spinning speed: Glycine Spectrometer edsp: NUC2 1 H Parameter set: CPGLY Pulse program: cp.av Parameters: Acquisition: PULPROG cp90.av TD 2048 SW 300ppm D1 5s Spin at N khz where N is defined by the 1H spectrometer frequency divided by 100MHz (e.g. for a MHz spectrometer N=5). NUC1 13 C NS 4 PL1 for 4 or 4.5 us pulse as determine in 4.4 P µs PL2 power level for us 1 H pulses minus 3 db SPNAM0 ramp.100 PL12 Power level for excitation pulse P3 and decoupling. Use 100% of the maximum values for sensitivity specs and 80% for the PROSOL Index: 04 Number: B92999 Page: 19 (42)
20 table (56kHz, 80 khz and 120 khz for 7, 4 and 2.5 mm probes)! Max. decoupling is 75 khz, 100 khz and 150 khz for 7 mm, 4mm and 2.5 mm MAS probes, respectively. Acquire one experiment with maximum decoupling and aq=50 ms to document power handling. P3 Use the maximum values, 2.5us, 3.3us and 2us for 4mm, 7mm and 2.5mm MAS probes, respectively. Measure the 80% values and put those into the PROSOL table, i.e. use 3us, 4.5 and 2.1 us for 4mm, 7mm and 2.5mm MAS probes, respectively. P31 2*P3-0.2us to be optimized O1P 100 ppm O2P CPDPRG2 Processing: LB 0 Hz 3.5 ppm (to be optimized in steps <=500 Hz tppm15 or SPINAL64 (cw for 300MHz and Lower field spectrometers) Note: If the carbonyl peak comes properly up at 176 ppm the O2 parameter above can be used. If not, set the carbonyl peak to 176 ppm. In order to get the right decoupler frequency, measure SFO1 at the carbonyl peak and multiply this number with Using popt with 400 Hz steps can also be used to find the best position for the decoupler frequency. For more advice, consult one of the references given above. Sensitivity is determined by measuring the signal height of the aliphatic peak and noise over a 10 ppm wide region. Use the au program sinocal. Signal region 50 ppm to 30 ppm, range for noise determination from 250 ppm to 50 ppm and noise 10 ppm. When using ATP the appropriate parameters for sinocal are readily available and sinocal loads those parameters automatically. Index: 04 Number: B92999 Page: 20 (42)
21 Acceptance Test Procedure Determination of 90 deg pulse for 13C poptau for p1 finished. ZERO at experiment : p1 = NEXP= ppm Index: 04 Number: B92999 Page: 21 (42)
22 C π/2 Pulse determination Test Sample: Spinning speed: Glycine Spectrometer edsp: NUC2 1 H Parameter set: CPGLY Pulse program: cp90.av Parameters: Acquisition: PULPROG cp90.av Set: Spin at N khz where N is defined by the 1H spectrometer frequency divided by 100MHz (e.g. for a MHz spectrometer N=5). NUC1 13 C PL11 = PL1 all other parameters used as in 4.6 Processing as above Note: Use popt for this experiment. A π/2 pulse on 13 C with the right phase after the spin lock gives no signal! See figure. Note: Enter the value pl11 and p1 into P90 of the prosol table. Index: 04 Number: B92999 Page: 22 (42)
23 Final Final Test Test Probe Probe B0628/101: PH MAS WB BL-4 1H/X/Y Done Done by: by: JOS JOS Glycine Glycine 15N 15N cp CP experiment experiments Spinning speed 5000 Hz After Spinning some optimization: speed 11000Hz S/N Signal from Signal to Noise: to ppm Noise Signal from from to to ppm ppm Noise from 179 to 129 ppm pp Index: 04 Number: B92999 Page: 23 (42)
24 N Sensitivity on Glycine Test sample: Spinning speed: Spectrometer edsp: Parameter set: Pulse program: Glycine, 15 N natural abundance For parameter optimization: use 15 N enriched Glycine Spin at N khz where N is defined by the 1H spectrometer frequency divided by 100MHz (e.g. for a MHz spectrometer N=5). NUC1 15 N NUC2 1 H CPN15 cp90.av Parameters: Acquisition: PULPROG cp90.av TD 2024 P1 5.5us ( goes with PL11) P15 3 ms SW 400ppm D1 5s RG 8096 PL1 Power level for 5 or 5.5 us pulse PL db PL2 power level for 5 or 5.5 us 1H pulse + 3dB SPNAM0 ramp.100 PL12 Use same decoupling as in previous experiment with 13 C O1P 0 ppm O2P 5 ppm optimize Note: A good starting point for the choice of power levels is to use the power level of the 13 C and subtract 3dB ( double the power) and add 3dB to the proton power level pl2 used for 1 H- 13 C CP- MAS experiment. The 15 N peak is approximately at 33 ppm if the reference procedure was used following chapter 4.1 or 4.4. For o2p use o2p from Glycine sensitivity experiment and add 5.5 ppm. The best decoupler position can also be found using popt with 400 Hz steps. In order to setup HH and all other parameters it is advantageous to use a 15 N labeled glycine, if available. Another good and easy sample is 15 N enriched ammonium nitrate (allows direct polarization almost without any decoupling). Sensitivity is determined by measuring the signal integral over the peak and noise over a 20-ppm wide region. Use the au program sinocal. Signal region = 35 ppm to 25 ppm, whole spectrum 300 ppm to -300 ppm and noise 50 ppm. Then the program will look for the best noise region and return the values for noise region, signal region and S/N. Index: 04 Number: B92999 Page: 24 (42)
25 N π/2 Pulse determination Use same parameters as in N Sensitivity on Glycine and measure the pulse width as described in C π/2 Pulse determination. Index: 04 Number: B92999 Page: 25 (42)
26 Acceptance Test Procedures Solid State NMR 31P SINO ppm Index: 04 Number: B92999 Page: 26 (42)
27 P Sensitivity Test sample: Spinning speed: Ammoniumdihydrogen Phosphate See Glycine experiments Spectrometer edsp: Parameter set: Pulse program: NUC1 NUC2 CPP31 cp.av 31 P 1 H Parameters: Acquisition: PULPROG cp90.av AQ 50 ms P15 5 ms SW 300 ppm D1 5s PL1 for 5 us pulse PL2 for 5 us 1H pulse + 3dB PL12 see 13 C sensitivity experiment P3 see 13 C sensitivity experiment P31 see 13 C sensitivity experiment O1P 0 ppm O2P 2.5 ppm Note: The 31 P peak is at 2.5 ppm. A good starting value can be obtained by adding 3 db to the power level of HH match for 13 C (power reduction by 50%), and using the same pl12.. Sensitivity is determined by measuring the signal level of the peak and noise over a 20-ppm wide region. Use the au program sinocal. Signal region = 15 ppm to -15 ppm, whole spectrum 125 ppm to -125 ppm and noise bandwidth is 20 ppm. Then the program will look for the best noise region and return the values for noise region, signal region and S/N P π/2 Pulse determination Use same parameters as in 4.10 and measure the pulse width as described in C π/2 Pulse determination Index: 04 Number: B92999 Page: 27 (42)
28 4.12 Double CP (DCP) experiment Setup using 13 C 15 N labeled glycine in center-packed rotor and rotate at 11 khz The Setup is done in three steps. Step one is CP parameter determination and measurement for 15 N, Step 2 is CP parameter determination and measurement for 13 C at 11 khz sample rotation and step 3 is the optimization of the second CP step in the DCP experiment. In edasp the signal should be routed for each experiment as needed for the final DCP experiment. The routing for the 15 N 1 H CP experiment is shown in figure 1. The DCP routing can be used for the 13 C 1 H CP setup as well and is shown in figure 2. Figure 1: Setup for 15 N 1 H CP experiment, can be obtained from routing as in figure 3 by checking the Switch F1/F3 button. Index: 04 Number: B92999 Page: 28 (42)
29 Figure 2: Routing for CP experiment with 13 C and the double CP experiment Even if the CP conditions are known, it is advantageous to determine the 90 pulse of the X and Y nucleus using cp90.av. The 90 degree pulse is found, where the signal crosses Zero (see 4.7 and 4.9). The knowledge of the 90 degree pulse allows calculating the right HH condition by: B1_ 15 N (Hz) = B1_ 13 C (Hz) + spinning rate in Hz. Run a CP experiment 13 C{ 1 H} with 16 scans as reference to measure DCP yield.. Call the pulse program doubcp1.av. Use all the parameters from CP experiments: Test sample: center-packed U 13 C 15 N enriched glycine Spinning speed: 11 khz Spectrometer edsp: NUC1 13C NUC2 1 H NUC3 15 N Parameter set: Pulse program: DCPNC doubcp1.av Parameters: Acquisition: PULPROG doubcp1.av Index: 04 Number: B92999 Page: 29 (42)
30 TD 2048 P ms P ms optimize start with 4ms SW 300 ppm D1 5s PL1 for 5 us pulse on 13 C PL2 for best 1 H power level of HH match 15 N{ 1 H} PL3 for 5 us pulse on 15 N (or best rf-field achieved) PL5 for 35 khz rf field on 15 N (calculate) PL12 see 13 C sensitivity experiment PL khz cw decoupling (depending on specs) P3 see 13 C sensitivity experiment P31 see 13 C sensitivity experiment O1P 50 ppm O2P 3.5 ppm O3P 40 ppm Write all know pulse parameters into the parameter set, pl1, pl2, pl3, pl12, pl13, p3 and p31. Then calculate pl5 to achieve a rf field of 36 khz and enter pl5 into parameter set. e.g. if pl3 is 0 db for 50 khz ( 5us 90º pulse ) we calculate: 20 log36 50 =-2.85dB; pl5=pl db, i.e. pl5 = -2.8 db is the power level to use. Of course this calculation requires that CORTAB is done. Note: pl1 is the power level for a 50 khz carbon pulse. Loading the shape spnam1 = tancn gives a tangential pulse at 50% of the full pulse amplitude, i.e. at 25 khz which is the exact Hartmann Hahn (HH) cross polarization condition, f rf ( 15 N) = f rf ( 13 C) + 1*f rot, with f rf the 15 N and 13 C spin nutation frequencies and f rot the sample rotation. This is called the +1 HH condition. If everything is setup carefully one should see a good double CP signal with 8 scans. Default is 16 scans, but for parameter optimization 4 scans suffice. Optimize pl1 in steps of 0.1dB for ± 0.5 db if a good signal was obtained, for ± 1 db if a poor DCP signal was obtained. Do the optimization on the alphatic carbon resonance and use the full phase cycle ns = 16 to exclude artifacts. Run one experiment with 16 scans when all parameters are fully optimized and compare the intensity of the aliphatic resonance with the aliphatic resonance from the 13 C{ 1 H} CPMAS experiment. Index: 04 Number: B92999 Page: 30 (42)
31 Figure 3: DCP optimization on PL1 in 0.1 db steps Tuning Stability Test (optional) Having setup DCP create a new experiment with edc, load pulse program doubcp1_stab.av change in eda to 2D experiment and set td to 1 8k, set pl12 to 100 khz decoupling, D1 = 5s, ns = 8 and start experiment for an overnight run. Figure 4: Double CP experiments in a hard instrument stability test over night with 8192 experiments 2 scans per experiment. Note the horizontal line from an arcing event. Index: 04 Number: B92999 Page: 31 (42)
32 Figure 5: Double CP experiments column extracted from above 2D data set. The signal amplitude varies between 90% and 100% except for the arcing event. The tuning stability is well within the acceptance limits (20% instability) HFX Probes: HF Setup General cannel setup for HFC experiments benefits from 19 F preamplifier module like for example the XBB19F 2HS module (figure 6) Figure 6: HFC experiment setup example with routing table and preamplifier selection. Index: 04 Number: B92999 Page: 32 (42)
33 FH HF experiment Use the pulse program hpdec.av. Use all the known parameters from CP experiments. And do 19 F and 1 H detect experiment with 1 H and 19 F decoupling experiments. Test sample: Spinning speed: PVDF 15 khz or 35 khz if possible Spectrometer edsp: NUC1 NUC2 19 F 1 H Parameter set: Pulse program: FH_dp hpdec.av Parameters: Acquisition: PULPROG hpdec.av TD 2048 DW 5us D1 5s PL1 for 3.1 us pulse on 19 F see probe specs PL12 for 80 khz 1 H decoupling O1P 100 ppm O2P 3.5 ppm C{ 19 F} CPMAS experiments Use all the known parameters from CP experiments and from above HF experiments. With 2.5 mm probes at high spinning speed, > 20 khz, use for the contact pulse spnam0 = ramp Test sample: Spinning speed: PVDF 15 khz or 35 khz if possible Spectrometer edsp: NUC1 NUC2 NUC3 13 C 19 F or 1 H 1 H or 19 F Parameter set: Pulse program: CPCF cp.av Parameters: Acquisition: PULPROG cp.av TD 2048 DW 5us D1 5s PL1 CP power level for 4 us pulse f1 Index: 04 Number: B92999 Page: 33 (42)
34 PL2 PL12 O1P O2P CP power level f2 for 80 khz 1 H decoupling / 19 F decoupling on f2 50 or 100 ppm ppm Set 19 F carrier frequency on one of the 19 F resonances and optimized HH condition having 13 C contact pulse at 62.5 khz for moderate spinning speeds < 20 khz and for high spinning speed on 50 khz. For 13 C{ 19 F} experiment with 19 F decoupling use cp.av pulse program. For 13 C{ 19 F} experiment with 1 H decoupling use cpfh1.av pulse program C{ 19 F - 1 H} CPMAS experiments Use all the known parameters from CP experiments and from above HF experiments. With 2.5 mm probes at high spinning speed use for the contact pulse spnam0 = ramp Test sample: Spinning speed: PVDF 15 khz or 35 khz if possible Spectrometer edsp: NUC1 NUC2 NUC3 13 C 19 F 1 H Parameter set: Pulse program: CPCFH cpfh.av or cpfh1.av Parameters: Acquisition: PULPROG cpfh.av or cpfh1.av TD 2048 DW 5us D1 5s PL1 CP power level for 4 us pulse f1 PL2 CP power level f2 PL12 for 80 khz 1 H decoupling / 19 F decoupling on f2 PL13 for 80 khz 19 F decoupling / 1 H decoupling on f3 O1P 50 or 100 ppm O2P ppm or 3.5ppm O3P 3.5ppm or ppm Set 19 F carrier frequency on one of the 19 F resonances and optimized HH condition having 13 C contact pulse at 62.5 khz for moderate spinning speeds < 20 khz and for high spinning speed on 50 khz. For 13 C{ 19 F} experiment with 1 H decoupling use cpfh1.av pulse program. For 13 C{ 19 F} experiment with 1 H and 19 F decoupling use cpfh.av pulse program. Index: 04 Number: B92999 Page: 34 (42)
35 C{ 1 H 19 F} CPMAS experiments Use all the known parameters from CP experiments and from above HF experiments. With 2.5 mm probes at high spinning speed use for the contact pulse spnam0 = ramp Test sample: Spinning speed: Spectrometer edsp: PVDF 15 khz NUC1 NUC2 NUC3 13 C 1 H 19 F Parameter set: Pulse program: CPCFH cpfh.av or cpfh1.av Parameters: Acquisition: PULPROG cpfh.av or cpfh1.av TD 2048 DW 5us D1 5s PL1 CP power level for 4 us pulse f1 PL2 CP power level f2 PL12 for 80 khz 1 H decoupling / 19 F decoupling on f2 PL13 for 80 khz 19 F decoupling / 1 H decoupling on f3 O1P 50 or 100 ppm O2P ppm or 3.5ppm O3P 3.5ppm or ppm Use optimized HH condition from 13 C sensitivity experiment. For 13 C{ 1 H} experiment with 19 F decoupling use cpfh1.av pulse program. For 13 C{ 1 H} experiment with 1 H and 19 F decoupling use cpfh.av pulse program. Set 19 F carrier frequency on one of the 19 F resonances. 7. Shimming a CP MAS probe When shimming a high-resolution liquids probe there is a distinction between on-axis (Z) and offaxis (X, Y etc.) shims. Spinning the sample parallel to the magnetic field direction averages the off-axis inhomogeneity, which may result in spinning sidebands. In MAS spectroscopy the spinner axis is at an angle, θ m with the magnetic field direction and the distinction between the traditional on-axis and off-axis shims no longer holds. The spinning rates in MAS spectroscopy, however, are typically at least a few kilohertz; much larger than the magnetic Index: 04 Number: B92999 Page: 35 (42)
36 field inhomogeneity. As a result, the amplitudes of the sidebands are small and shimming may be done with a set of shims that is cylindrically symmetric about the MAS spinner axis. Such a set of shims can be constructed from combinations of the standard laboratory frame shims, via a transformation to the tilted magic angle frame. In the simplest implementation, the MAS probe is aligned such that the spinner axis is in the laboratory YZ-plane which holds for WB probes in Figure 1 the configuration for SB probes is shown, with the alignment in the XY-plane. The 'magic angle z-shims' are listed below for orders Z through Z 5 (Sodickson and Cory 1997). The simplest approach is to use the laboratory shims with the largest coefficient, so for instance the Y shim is used as the normal high resolution Z shim. Complete shimming can then in principle be accomplished using only Y, ZY, Z 2 Y, Z 4 and Z 5 as the surrogate n th order on-axis shims. Note, however, that the coefficients given for the magic angle shims do not take into account the efficiencies of the shim coils and the use of the other shims listed below may be needed. z y shim plate leads x Figure 1: TFor HRMAS probes and BioSolids Probes, the magic angle spinner axis is aligned with the xz-plane of the shims by positioning the front plate of the probe parallel to the direction of the shim leads. For WB probes, the positioning of the MAS rotor is by 90º shifted, and the long axis is parallel to the front plate. The y-direction of the shim system is parallel to the direction of the leads / the long side of the RT-shim base plate. Index: 04 Number: B92999 Page: 36 (42)
37 MAS shims Z m Z m 2 Laboratory shims 1 2 Z Y 3 3 (X 2 - Y 2 ) 2 2 ZY Z 3 m Z 3 Z 2 Y + (X 2 Y 2 )Z - Y Z 4 m Z4 Z m Z5 To shim the MAS probe, a sample of adamantane is suggested. Make sure the magic angle is adjusted before shimming the probe. Spin the sample at a rate suggested in the following section. It is always best to shim up the probe already under the conditions used later for the real samples. See also chapter 4.4 and 4.5 for discussion regarding the use of direct polarization or cross polarization to shim. Cross polarization is needed if shimming a 2.5 mm CPMAS probe as it gives more signal intensity. Alternatively, make use of a lock control board if available, fill the rotor with D 2 O and lock through the X channel on D 2 O. Then, shimming on the lock signal is easily possible. Lock the sample if D 2 O is used, tune the probe and start shimming using the Y, ZY, Z 2 Y, Z 4 and Z 5 shims as described above. If one of the shims requires an excessive shim current, reduce the current and continue shimming by adding current to another shim from the same group as shown in the table. For instance if the current in ZX is too high, reduce the value and optimize the line shape by adding current to the (X 2 -Y 2 )Z shim. If the probe is not exactly aligned with the xz plane (see Figure 1), then a small amount of X and XZ may be needed for optimal shimming. A second shim set should be obtained for rotors with spacers (CRAMPS or hr-mas rotors), and the optimal shim settings for these will be somewhat different. Under normal conditions the shim values obtained as described above, will be close to optimal for all other samples. When changing samples, only Y, Z (and ZY) need to be adjusted. Both shim value sets should be stored on disk. Make sure when the shim values are stored that the MAS probe is defined as the current probe in the EDHEAD command. Note: The alignment of the spinner axis in hr-mas probes is shifted by 90 degrees. The spinner lies in the XZ plane. Through symmetry, the appropriate shims to use are now X, Z, XZ, XZ 2 etc.. See table 3 in the reference. Reference: A. Sodickson and D.G. Cory, Shimming a High-Resolution MAS Probe, J. Magn. Reson. 128, (1997) Index: 04 Number: B92999 Page: 37 (42)
38 8. Prosol table and relations file for CP MAS experiments 1 H Pulses and power levels entry: Prepare the prosol table for the CPMAS use by entering the ramped pulse into the table to the decoupler channel (F2) with the shaped pulses for PSH16, last row, last column for nucleus 1 H (see figure). Index: 04 Number: B92999 Page: 38 (42)
39 Then return to the standard hard pulses. Write pulses and power levels into the prosol table as usual for the 1 H experiment with adamantane into F1. Use for the decoupling pulse a pulse width of 120% as compared to the maximum possible decoupling field, i.e. for a 4mm WB CPMAS probe this would be 2.5us (100 khz) for the maximum and then 3.1us (80kHz) for the entry into the field PCPDP. Enter the obtained value for the power level also into the next field for PLCPD2. If P3 is measured during the CP experiments with glycine, this value is entered as P90 into the appropriate field on the right block, F2, with the power level from PL12 of the experiment, 1H on channel F2 routed to amplifier A2. The power level for the contact pulse is calculated in the field PLNOE using Hz. The adjustment for the ramped contact pulse is done in the prosol file automatically during loading! Index: 04 Number: B92999 Page: 39 (42)
40 13 C pulses and power levels: Enter the value for the 4us 90 degree pulse into the field for P90 and calculate the HH match power level for Hz in the field PLCW. 9. Listings: Relations file, pulse program:cp.av.xyz #******************************************************************* # # $Source: /sc/cvstree/sc/gen/src/prg/scripts/tix/prosol/lib/lists/solids_cp,v $ # # Copyright (c) 1999 # BRUKER ANALYTIK GMBH # D Rheinstetten, Germany # # All Rights Reserved # Index: 04 Number: B92999 Page: 40 (42)
41 # # $Id: solids_cp,v /04/26 14:48:33 bau Exp $ # #******************************************************************* #../conf/instr/spect/prosol/relations/solids_cp P[0]=P90[F1]; P[1]=P90[F1]; # 90 degree pulse at power level pl11 for x or y # nucleus P[2]=P90[F1]*2; # 180 degree pulse at pl11 P[3]=PCPDP[F2]; # 90 degree pulse on 1H at pl12 P[4]=P90[F2]*2; # 180 degree pulse on 1H at pl12 P[11]=P90[F3]; # 90 degree pulse on F3 P[12]=P90[F3]*2; # 180 degree pulse for REDOR on F3 P[22]=P90[F2]*2; # 180 degree pulse for pi decoupling P[31]=PCPDP[F2]*2-0.2u; # TPPM decoupling pulse width PL[1]=PLCW[F1]; # power level for HH contact pulse F1 set to 62.5kHz PL[2]=PLNOE[F2]-3; # power level for HH contact pulse F2 62.5kHz -3dB as # we use ramp.100 in parameter set which is a 6dB # amplitude change # set PLNOE to 62.5kHz PL[3]=PL90[F3]; # power level for pulse on F3 PL[11]=PL90[F1]; # power level for hard pulse F1 PL[12]=PLCPDP[F2]; # set PLCPD to 80 khz (3.1us pulse with for 4mm probe #(80% of max. possible decoupling field PL[13]=PLHD[F2]; # power level for homonuclear decoupling, e.g FSLG set # to 80 khz SPNAM0=PNSH16[F2]; # set to ramp.100 as default for 1H on F2 P[15]=P_grad1*2; # HH contact pulse width for 1H-X polarization transfer ;cp.av prosol relations=<solids_cp> #include <lgcalc.incl> ;cnst20=rf field achieved at pl13 ;cnst24:additional LG-offset #include <trigg.incl> ;10 usec trigger pulse at TCU connector I cable 6 #include <tppm.incl> Index: 04 Number: B92999 Page: 41 (42)
42 1 ze ;accumulate into an empty memory if "aq < 1.0s" goto 2 print "acquisition time exceeds 1s time limit!" goto HaltAcqu 2 d1 do:f2 ;recycle delay, decoupler off in go-loop #include <prp15.prot> ;make sure p15 does not exceed 10 msec ;let supervisor change this pulseprogram if ;more is needed #ifndef lacq /* disable protection file for long acquisition change decoupling power!!! or you risk probe damage */ /* if you set the label lacq (ZGOPTNS -Dlacq), the protection is disabled */ #include <praq.prot> ;allows max. 50 msec acquisition time, supervisor ;may change to max. 1s at less than 5 % duty cycle ;and reduced decoupling field #endif 1u fq=cnst21:f2 10u pl12:f2 pl1:f1 ;preselect pl12 drive power for F2, pl1 for F1 trigg ;trigger for scope, 10 usec p3:f2 ph1 ;proton 90 pulse 0.3u (p15 ph2):f1 (p15:spf0 pl2 ph10):f2 ;contact pulse with square or ramp ;shape on F2, at pl2 proton power level 1u cpds2:f2 go=2 ph31 1m do:f2 wr #0 HaltAcqu, 1m exit ;select appropriate decoupling sequence, cw or ;tppm, both executed at power level 12, or lgs ;executed at power level pl13 ;decoupler off ;save data to disk ;jump address for protection files ;quit ph0= 0 ph1= 1 3 ph2= ph10= 0 ph31= Index: 04 Number: B92999 Page: 42 (42)
Principios Básicos de RMN en sólidos destinado a usuarios. Gustavo Monti. Fa.M.A.F. Universidad Nacional de Córdoba Argentina
 Principios Básicos de RMN en sólidos destinado a usuarios Gustavo Monti Fa.M.A.F. Universidad Nacional de Córdoba Argentina magnet 1 2 4 5 6 computer 3 Block diagrama of a traditional NMR spectrometer.
Principios Básicos de RMN en sólidos destinado a usuarios Gustavo Monti Fa.M.A.F. Universidad Nacional de Córdoba Argentina magnet 1 2 4 5 6 computer 3 Block diagrama of a traditional NMR spectrometer.
HMBC 17. Goto. Introduction AVANCE User s Guide Bruker 185
 Chapter HMBC 17 Introduction 17.1 Goto Heteronuclear Multiple Bond Correlation spectroscopy is a modified version of HMQC suitable for determining long-range 1 H- 13 C connectivity. This is useful in determining
Chapter HMBC 17 Introduction 17.1 Goto Heteronuclear Multiple Bond Correlation spectroscopy is a modified version of HMQC suitable for determining long-range 1 H- 13 C connectivity. This is useful in determining
User manual Bruker DPX200 NMR spectrometer
 User manual Bruker DPX200 NMR spectrometer Insert the NMR tube in the spinner in such a way that the bottom of the tube reaches the grey disc at the bottom of the spinnerholder. Make sure that the NMR
User manual Bruker DPX200 NMR spectrometer Insert the NMR tube in the spinner in such a way that the bottom of the tube reaches the grey disc at the bottom of the spinnerholder. Make sure that the NMR
Two Dimensional Homonuclear Correlation Spectroscopy
 Two Dimensional Homonuclear Correlation Spectroscopy Gradient COSY William D. Wheeler, Ph.D. Department of Chemistry University of Wyoming April 16, 1999 Revised September 22, 1999 2 INTRODUCTION Correlation
Two Dimensional Homonuclear Correlation Spectroscopy Gradient COSY William D. Wheeler, Ph.D. Department of Chemistry University of Wyoming April 16, 1999 Revised September 22, 1999 2 INTRODUCTION Correlation
H Micro-Imaging. Tuning and Matching. i. Open any 1H data set and type wobb.
 - 1-1 H Micro-Imaging The NMR-specific properties of the objects are visualized as multidimensional images. Translational motion can be observed and spectroscopic information can be spatially resolved.
- 1-1 H Micro-Imaging The NMR-specific properties of the objects are visualized as multidimensional images. Translational motion can be observed and spectroscopic information can be spatially resolved.
NMR Basics. Lecture 2
 NMR Basics Lecture 2 Continuous wave (CW) vs. FT NMR There are two ways of tuning a piano: - key by key and recording each sound (or frequency). - or, kind of brutal, is to hit with a sledgehammer and
NMR Basics Lecture 2 Continuous wave (CW) vs. FT NMR There are two ways of tuning a piano: - key by key and recording each sound (or frequency). - or, kind of brutal, is to hit with a sledgehammer and
Implementing ultrafast 2D NMR experiments on a Bruker Avance Spectrometer
 Implementing ultrafast 2D NMR experiments on a Bruker Avance Spectrometer Laetitia Rouger, Benoît Charrier, Serge Akoka, Patrick Giraudeau EBSI group CEISAM laboratory http://www.sciences.univ-nantes.fr/ceisam/en_ebsi1.php
Implementing ultrafast 2D NMR experiments on a Bruker Avance Spectrometer Laetitia Rouger, Benoît Charrier, Serge Akoka, Patrick Giraudeau EBSI group CEISAM laboratory http://www.sciences.univ-nantes.fr/ceisam/en_ebsi1.php
Step by step procedure for NMR data acquisition
 Step by step procedure for NMR data acquisition Spectrometers The UTHSCSA 500, 600, and 700 MHz spectrometers are each equipped with 4 independent RF channels and are each operated by a Red Hat Linux workstation
Step by step procedure for NMR data acquisition Spectrometers The UTHSCSA 500, 600, and 700 MHz spectrometers are each equipped with 4 independent RF channels and are each operated by a Red Hat Linux workstation
1D Transient NOE on the Bruker DRX-500 and DRX-600
 1D Transient NOE on the Bruker DRX-500 and DRX-600 Reference: Stott, K., Stonehouse, J., Keeler, T.L. and Shaka, A.J., J. Amer. Chem. Soc. 1995, 117 (14), pp. 4199-4200. At thermal equilibrium in a strong
1D Transient NOE on the Bruker DRX-500 and DRX-600 Reference: Stott, K., Stonehouse, J., Keeler, T.L. and Shaka, A.J., J. Amer. Chem. Soc. 1995, 117 (14), pp. 4199-4200. At thermal equilibrium in a strong
Two Dimensional Homonuclear Correlation Spectroscopy
 Two Dimensional Homonuclear Correlation Spectroscopy DQF-COSY William D. Wheeler, Ph.D. Department of Chemistry University of Wyoming September 23, 1999 2 INTRODUCTION Correlation Spectroscopy Correlation
Two Dimensional Homonuclear Correlation Spectroscopy DQF-COSY William D. Wheeler, Ph.D. Department of Chemistry University of Wyoming September 23, 1999 2 INTRODUCTION Correlation Spectroscopy Correlation
Ultrahigh-resolution Total Correlation NMR Spectroscopy
 Ultrahigh-resolution Total Correlation NMR Spectroscopy Supporting Information Mohammadali Foroozandeh, Ralph W. Adams, Mathias Nilsson and Gareth A. Morris* All experimental spectra were recorded at a
Ultrahigh-resolution Total Correlation NMR Spectroscopy Supporting Information Mohammadali Foroozandeh, Ralph W. Adams, Mathias Nilsson and Gareth A. Morris* All experimental spectra were recorded at a
Two Dimensional Heteronuclear Correlation Spectroscopy
 Two Dimensional Heteronuclear Correlation Spectroscopy Gradient HMQC William D. Wheeler, Ph.D. Department of Chemistry University of Wyoming Revised September 7, 2006 2 INTRODUCTION Correlation Spectroscopy
Two Dimensional Heteronuclear Correlation Spectroscopy Gradient HMQC William D. Wheeler, Ph.D. Department of Chemistry University of Wyoming Revised September 7, 2006 2 INTRODUCTION Correlation Spectroscopy
NMR Spectrometer Operation: xwinnmr
 NMR Spectrometer Operation: xwinnmr Dr. Robert Peterson Facility Manager NMR Technology Center UCLA-DOE Institute for Genomics and Proteomics UCLA Dept. of Chemistry and Biochemistry Overview This is a
NMR Spectrometer Operation: xwinnmr Dr. Robert Peterson Facility Manager NMR Technology Center UCLA-DOE Institute for Genomics and Proteomics UCLA Dept. of Chemistry and Biochemistry Overview This is a
Your first NMR measurement
 Your first NMR measurement Introduction Select 10mM water in D2O as NMR sample. The NMR spectrum of such sample consists of only two signals: the water signal and the peak of the reference (TSP). Follow
Your first NMR measurement Introduction Select 10mM water in D2O as NMR sample. The NMR spectrum of such sample consists of only two signals: the water signal and the peak of the reference (TSP). Follow
Chapter 1. 1 The NMR Spectrometer. 1.1 Components of an NMR Spectrometer The Magnet
 Chapter 1 1 The NMR Spectrometer 1.1 Components of an NMR Spectrometer 1.1.1 The Magnet In most current NMR spectrometers the magnetic field is generated by a superconducting magnet (Fig. 1.1). The first
Chapter 1 1 The NMR Spectrometer 1.1 Components of an NMR Spectrometer 1.1.1 The Magnet In most current NMR spectrometers the magnetic field is generated by a superconducting magnet (Fig. 1.1). The first
Student Name: Date Completed: Supervisor:
 2 NMR Training for the 600 MHz NMR with Chempack INOVA 600 Tests and Assignment Certification Student Name: 600-Test #1: The student will be given a written test administered by Dr. Lee. This test will
2 NMR Training for the 600 MHz NMR with Chempack INOVA 600 Tests and Assignment Certification Student Name: 600-Test #1: The student will be given a written test administered by Dr. Lee. This test will
Laboratory Experiments for Nuclear Magnetic Resonance Spectroscopy May 6, 2004
 CONTENTS 1 Contents Laboratory Experiments for Nuclear Magnetic Resonance Spectroscopy May 6, 2004 1 Introduction 3 2 Safety 3 2.1 High Magnetic Fields......................................... 3 2.1.1
CONTENTS 1 Contents Laboratory Experiments for Nuclear Magnetic Resonance Spectroscopy May 6, 2004 1 Introduction 3 2 Safety 3 2.1 High Magnetic Fields......................................... 3 2.1.1
400 MHz spectrometer user manual
 400 MHz spectrometer user manual january 2017 Sandrine Denis-Quanquin 1. THE NMR SPECTROMETER... 3 2. MANUAL MODE / AUTOMATION... 4 2.1 SAMPLE CHANGER... 4 2.2 MANUAL MODE... 4 2.3 AUTOMATION... 4 3. PRELIMINARY
400 MHz spectrometer user manual january 2017 Sandrine Denis-Quanquin 1. THE NMR SPECTROMETER... 3 2. MANUAL MODE / AUTOMATION... 4 2.1 SAMPLE CHANGER... 4 2.2 MANUAL MODE... 4 2.3 AUTOMATION... 4 3. PRELIMINARY
PHY3902 PHY3904. Nuclear magnetic resonance Laboratory Protocol
 PHY3902 PHY3904 Nuclear magnetic resonance Laboratory Protocol PHY3902 PHY3904 Nuclear magnetic resonance Laboratory Protocol GETTING STARTED You might be tempted now to put a sample in the probe and try
PHY3902 PHY3904 Nuclear magnetic resonance Laboratory Protocol PHY3902 PHY3904 Nuclear magnetic resonance Laboratory Protocol GETTING STARTED You might be tempted now to put a sample in the probe and try
NMR Hardware 06/06/2017. Outline. Instrumentation: Magnet. Increasing magnetic field increases Sensitivity, by power of 3/2 Dispersion, linearly
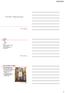 NMR Hardware Outline Magnet Lock Shims Gradient Probe Signal generation and transmitters Receiver and digitizer Variable temperature system Solids hardware Instrumentation: Magnet Often the most impressive
NMR Hardware Outline Magnet Lock Shims Gradient Probe Signal generation and transmitters Receiver and digitizer Variable temperature system Solids hardware Instrumentation: Magnet Often the most impressive
8 COSY. 8.1 Introduction. 8.2 Magnitude COSY
 8 COSY 8.1 Introduction COSY (COrrelation SpectroscopY) is a homonuclear 2D technique that is used to correlate the chemical shifts of 1 H nuclei which are J-coupled to one another. In this chapter, two
8 COSY 8.1 Introduction COSY (COrrelation SpectroscopY) is a homonuclear 2D technique that is used to correlate the chemical shifts of 1 H nuclei which are J-coupled to one another. In this chapter, two
Instruction for Operating the Bruker Avance III 800 MHz NMR Spectrometers in UTMB
 Instruction for Operating the Bruker Avance III 800 MHz NMR Spectrometers in UTMB Written by Tianzhi Wang, date: February 8, 2013. No food, no drink in NMR room and no internet in NMR host computer except
Instruction for Operating the Bruker Avance III 800 MHz NMR Spectrometers in UTMB Written by Tianzhi Wang, date: February 8, 2013. No food, no drink in NMR room and no internet in NMR host computer except
Open acqi window if the button has been lost. autolocking routine, alock= y for autolocking, alock= n for typical manual locking
 Glossary of Common NMR Commands and Terms aa acqi ai alock aph array at points (np) axis='p' axis= pd BPsvf bc bs cd directory abort acquisition, hard stop Open acqi window if the button has been lost
Glossary of Common NMR Commands and Terms aa acqi ai alock aph array at points (np) axis='p' axis= pd BPsvf bc bs cd directory abort acquisition, hard stop Open acqi window if the button has been lost
NMR Spectroscopy with Radio Frequency Gradients.
 RF GRASP TM NMR Spectroscopy with Radio Frequency Gradients. BRUKER Werner E. Maas Bruker Instruments, Inc. 19 Fortune Drive Billerica, MA 01821 USA version 1.2 February, 1996 Copyright 1996 Bruker Instruments,
RF GRASP TM NMR Spectroscopy with Radio Frequency Gradients. BRUKER Werner E. Maas Bruker Instruments, Inc. 19 Fortune Drive Billerica, MA 01821 USA version 1.2 February, 1996 Copyright 1996 Bruker Instruments,
KJM Version 1.0. Topspin 3.5 Windows 7 Topspin 1.3 Windows XP
 KJM 9250 1 H NMR spectra on the AVI-600 and AVII-600 Version 1.0 Topspin 3.5 Windows 7 Topspin 1.3 Windows XP Professor Emeritus Alistair Lawrence Wilkins, University of Waikato, New Zealand. January 2018
KJM 9250 1 H NMR spectra on the AVI-600 and AVII-600 Version 1.0 Topspin 3.5 Windows 7 Topspin 1.3 Windows XP Professor Emeritus Alistair Lawrence Wilkins, University of Waikato, New Zealand. January 2018
KJM D-SELECTIVE NMR Experiments on the AVIIIHD-800. Version 1.0. Topspin 3.5 Windows 7
 KJM 9250 1D-SELECTIVE NMR Experiments on the AVIIIHD-800 Version 1.0 Topspin 3.5 Windows 7 Professor Emeritus Alistair Lawrence Wilkins, University of Waikato, New Zealand. January 2018 1D-SELECTIVE NMR
KJM 9250 1D-SELECTIVE NMR Experiments on the AVIIIHD-800 Version 1.0 Topspin 3.5 Windows 7 Professor Emeritus Alistair Lawrence Wilkins, University of Waikato, New Zealand. January 2018 1D-SELECTIVE NMR
NMR spectrometer usage at the BioNMR facility ETH Zürich
 NMR spectrometer usage at the BioNMR facility ETH Zürich Accounts 2 Safety precautions: strong magnetic fields 2 Parts of an NMR spectrometer 3 NMR data storage 3 Start topspin software 3 Initial steps
NMR spectrometer usage at the BioNMR facility ETH Zürich Accounts 2 Safety precautions: strong magnetic fields 2 Parts of an NMR spectrometer 3 NMR data storage 3 Start topspin software 3 Initial steps
KJM D-SELECTIVE NMR Experiments on the AVI-600 and AVII-600. Version 1.0. Topspin 3.5 Windows 7 Topspin 1.3 Windows XP
 KJM 9250 1D-SELECTIVE NMR Experiments on the AVI-600 and AVII-600 Version 1.0 Topspin 3.5 Windows 7 Topspin 1.3 Windows XP Professor Emeritus Alistair Lawrence Wilkins, University of Waikato, New Zealand.
KJM 9250 1D-SELECTIVE NMR Experiments on the AVI-600 and AVII-600 Version 1.0 Topspin 3.5 Windows 7 Topspin 1.3 Windows XP Professor Emeritus Alistair Lawrence Wilkins, University of Waikato, New Zealand.
Relaxation-encoded NMR experiments for mixture analysis: REST and beer. Electronic Supporting Information
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Relaxation-encoded NMR experiments for mixture analysis: REST and beer Electronic Supporting Information
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Relaxation-encoded NMR experiments for mixture analysis: REST and beer Electronic Supporting Information
Fast Methods for Small Molecules
 Fast Methods for Small Molecules Technical Overview Throughput is a key concern in many NMR laboratories, and using faster methods is one way to increase it. Traditionally, multidimensional NMR requires
Fast Methods for Small Molecules Technical Overview Throughput is a key concern in many NMR laboratories, and using faster methods is one way to increase it. Traditionally, multidimensional NMR requires
10. Phase Cycling and Pulsed Field Gradients Introduction to Phase Cycling - Quadrature images
 10. Phase Cycling and Pulsed Field Gradients 10.1 Introduction to Phase Cycling - Quadrature images The selection of coherence transfer pathways (CTP) by phase cycling or PFGs is the tool that allows the
10. Phase Cycling and Pulsed Field Gradients 10.1 Introduction to Phase Cycling - Quadrature images The selection of coherence transfer pathways (CTP) by phase cycling or PFGs is the tool that allows the
SUPPORTING INFORMATION
 Eur. J. Org. Chem. 2008 WILEY-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2008 ISSN 1434 193X SUPPORTING INFORMATION Title: Structural Elucidation with NMR Spectroscopy: Practical Strategies for Organic
Eur. J. Org. Chem. 2008 WILEY-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2008 ISSN 1434 193X SUPPORTING INFORMATION Title: Structural Elucidation with NMR Spectroscopy: Practical Strategies for Organic
2015 Spin echoes and projection imaging
 1. Spin Echoes 1.1 Find f0, transmit amplitudes, and shim settings In order to acquire spin echoes, we first need to find the appropriate scanner settings using the FID GUI. This was all done last week,
1. Spin Echoes 1.1 Find f0, transmit amplitudes, and shim settings In order to acquire spin echoes, we first need to find the appropriate scanner settings using the FID GUI. This was all done last week,
Chem 203. Organic Spectroscopy. Midterm Examination, Part II (60 points total) Problem 4 of 4 (three out of four required, 20 points)
 NAME Chem 203 Organic Spectroscopy Midterm Examination, Part II (60 points total) Problem 4 of 4 (three out of four required, 20 points) Saturday, November 15, 2014, 9 am -??? SUBMIT THREE OF THE FOUR
NAME Chem 203 Organic Spectroscopy Midterm Examination, Part II (60 points total) Problem 4 of 4 (three out of four required, 20 points) Saturday, November 15, 2014, 9 am -??? SUBMIT THREE OF THE FOUR
Temps can range -130 to +120 C. See instructions below (part I). Such data can also be acquired on Athena and the AVANCE-360.
 Flourine-19 Experiments on Varian Spectrometers updated: 2010 May 12 The difficulty with 19 F experiments involving decoupling (or 2D heterocorrelation) is the close proximity of the 19 F resonant frequency
Flourine-19 Experiments on Varian Spectrometers updated: 2010 May 12 The difficulty with 19 F experiments involving decoupling (or 2D heterocorrelation) is the close proximity of the 19 F resonant frequency
Chem 203. Organic Spectroscopy. Midterm Examination, Part II (60 points total) Problem 1 of 4 (three out of four required, 20 points)
 NAME Chem 203 Organic Spectroscopy Midterm Examination, Part II (60 points total) Problem 1 of 4 (three out of four required, 20 points) Saturday, November 15, 2014, 9 am -??? SUBMIT THREE OF THE FOUR
NAME Chem 203 Organic Spectroscopy Midterm Examination, Part II (60 points total) Problem 1 of 4 (three out of four required, 20 points) Saturday, November 15, 2014, 9 am -??? SUBMIT THREE OF THE FOUR
B12. SIMPSON Exercises
 B12 SIMPSON Exercises Thomas Vosegaard SIMPSON may be downloaded from http://www.bionmr.chem.au.dk/download/b12. SIMPSON Exercises Everything typed in Courier refers to commands to be typed on the keyboard
B12 SIMPSON Exercises Thomas Vosegaard SIMPSON may be downloaded from http://www.bionmr.chem.au.dk/download/b12. SIMPSON Exercises Everything typed in Courier refers to commands to be typed on the keyboard
Chem 203. Organic Spectroscopy. Midterm Examination, Part II (60 points total) Problem 4 of 4 (three out of four required, 20 points)
 NAME Chem 203 Organic Spectroscopy Midterm Examination, Part II (60 points total) Problem 4 of 4 (three out of four required, 20 points) Saturday, November 9, 2013, 9 am -??? SUBMIT THREE OF THE FOUR PROBLEMS
NAME Chem 203 Organic Spectroscopy Midterm Examination, Part II (60 points total) Problem 4 of 4 (three out of four required, 20 points) Saturday, November 9, 2013, 9 am -??? SUBMIT THREE OF THE FOUR PROBLEMS
Frequency and Time Domain Representation of Sinusoidal Signals
 Frequency and Time Domain Representation of Sinusoidal Signals By: Larry Dunleavy Wireless and Microwave Instruments University of South Florida Objectives 1. To review representations of sinusoidal signals
Frequency and Time Domain Representation of Sinusoidal Signals By: Larry Dunleavy Wireless and Microwave Instruments University of South Florida Objectives 1. To review representations of sinusoidal signals
Chem 203 December 15, Final Exam Part II Problem 3 of 3 (30 points)
 Name: Chem 203 December 15, 2012 Final Exam Part II Problem 3 of 3 (30 points) Select and submit TWO OUT OF THE THREE PROBLEMS FROM PART II for grading. Do not submit three problems. If you wish to unstaple
Name: Chem 203 December 15, 2012 Final Exam Part II Problem 3 of 3 (30 points) Select and submit TWO OUT OF THE THREE PROBLEMS FROM PART II for grading. Do not submit three problems. If you wish to unstaple
(N)MR Imaging. Lab Course Script. FMP PhD Autumn School. Location: C81, MRI Lab B0.03 (basement) Instructor: Leif Schröder. Date: November 3rd, 2010
 (N)MR Imaging Lab Course Script FMP PhD Autumn School Location: C81, MRI Lab B0.03 (basement) Instructor: Leif Schröder Date: November 3rd, 2010 1 Purpose: Understanding the basic principles of MR imaging
(N)MR Imaging Lab Course Script FMP PhD Autumn School Location: C81, MRI Lab B0.03 (basement) Instructor: Leif Schröder Date: November 3rd, 2010 1 Purpose: Understanding the basic principles of MR imaging
PINMRF. Varian 300 MHz NMR Spectrometers User Guide for Advanced 1D and Basic 2D NMR Experiments
 PINMRF Varian 300 MHz NMR Spectrometers User Guide for Advanced 1D and Basic 2D NMR Experiments INCLUDING: Inova-300-1 w/ 5mm 4-nucleus probe 365 WTHR Inova-300-2 w/ 5mm 4-nucleus probe 4100 BRWN Table
PINMRF Varian 300 MHz NMR Spectrometers User Guide for Advanced 1D and Basic 2D NMR Experiments INCLUDING: Inova-300-1 w/ 5mm 4-nucleus probe 365 WTHR Inova-300-2 w/ 5mm 4-nucleus probe 4100 BRWN Table
Signal Generators for Anritsu RF and Microwave Handheld Instruments
 Measurement Guide Signal Generators for Anritsu RF and Microwave Handheld Instruments BTS Master Spectrum Master Tracking Generator Option 20 Vector signal Generator Option 23 Anritsu Company 490 Jarvis
Measurement Guide Signal Generators for Anritsu RF and Microwave Handheld Instruments BTS Master Spectrum Master Tracking Generator Option 20 Vector signal Generator Option 23 Anritsu Company 490 Jarvis
Gradients. Effects of B0 gradients on transverse magnetisation Similar to figure 10 of Sattler review Progr. NMR 34 (1999), 93
 Gradients 1. What are gradients? Modern high-resolution NMR probes contain -besides the RF coils - additional coils that can be fed a DC current. The coils are built so that a pulse (~1 ms long) of DC
Gradients 1. What are gradients? Modern high-resolution NMR probes contain -besides the RF coils - additional coils that can be fed a DC current. The coils are built so that a pulse (~1 ms long) of DC
PN9000 PULSED CARRIER MEASUREMENTS
 The specialist of Phase noise Measurements PN9000 PULSED CARRIER MEASUREMENTS Carrier frequency: 2.7 GHz - PRF: 5 khz Duty cycle: 1% Page 1 / 12 Introduction When measuring a pulse modulated signal the
The specialist of Phase noise Measurements PN9000 PULSED CARRIER MEASUREMENTS Carrier frequency: 2.7 GHz - PRF: 5 khz Duty cycle: 1% Page 1 / 12 Introduction When measuring a pulse modulated signal the
Lab 8 6.S02 Spring 2013 MRI Projection Imaging
 1. Spin Echos 1.1 Find f0, TX amplitudes, and shim settings In order to acquire spin echos, we first need to find the appropriate scanner settings using the FID GUI. This was all done last week, but these
1. Spin Echos 1.1 Find f0, TX amplitudes, and shim settings In order to acquire spin echos, we first need to find the appropriate scanner settings using the FID GUI. This was all done last week, but these
MT-540. D-Ribavirin, [ 3 H]- Lot A DG
![MT-540. D-Ribavirin, [ 3 H]- Lot A DG MT-540. D-Ribavirin, [ 3 H]- Lot A DG](/thumbs/85/92603848.jpg) MT-54 D-Ribavirin, [ 3 H]- Lot 165-145-225-A-2835-DG A) All chromatograms were run using the HPLC method described on the Product Data Sheet. Concentrations and volumes: Standard solution concentration
MT-54 D-Ribavirin, [ 3 H]- Lot 165-145-225-A-2835-DG A) All chromatograms were run using the HPLC method described on the Product Data Sheet. Concentrations and volumes: Standard solution concentration
Notes on OR Data Math Function
 A Notes on OR Data Math Function The ORDATA math function can accept as input either unequalized or already equalized data, and produce: RF (input): just a copy of the input waveform. Equalized: If the
A Notes on OR Data Math Function The ORDATA math function can accept as input either unequalized or already equalized data, and produce: RF (input): just a copy of the input waveform. Equalized: If the
PXA Configuration. Frequency range
 Keysight Technologies Making Wideband Measurements Using the Keysight PXA Signal Analyzer as a Down Converter with Infiniium Oscilloscopes and 89600 VSA Software Application Note Introduction Many applications
Keysight Technologies Making Wideband Measurements Using the Keysight PXA Signal Analyzer as a Down Converter with Infiniium Oscilloscopes and 89600 VSA Software Application Note Introduction Many applications
MAKING TRANSIENT ANTENNA MEASUREMENTS
 MAKING TRANSIENT ANTENNA MEASUREMENTS Roger Dygert, Steven R. Nichols MI Technologies, 1125 Satellite Boulevard, Suite 100 Suwanee, GA 30024-4629 ABSTRACT In addition to steady state performance, antennas
MAKING TRANSIENT ANTENNA MEASUREMENTS Roger Dygert, Steven R. Nichols MI Technologies, 1125 Satellite Boulevard, Suite 100 Suwanee, GA 30024-4629 ABSTRACT In addition to steady state performance, antennas
PULSED/CW NUCLEAR MAGNETIC RESONANCE
 PULSED/CW NUCLEAR MAGNETIC RESONANCE The Second Generation of TeachSpin s Classic Explore NMR for both Hydrogen (at 21 MHz) and Fluorine Nuclei Magnetic Field Stabilized to 1 part in 2 million Homogenize
PULSED/CW NUCLEAR MAGNETIC RESONANCE The Second Generation of TeachSpin s Classic Explore NMR for both Hydrogen (at 21 MHz) and Fluorine Nuclei Magnetic Field Stabilized to 1 part in 2 million Homogenize
Program. short intro the hardware locking and shimming 1D proton setup presaturation spectra
 Program short intro the hardware locking and shimming 1D proton setup presaturation spectra 13 C spectra, DEPT processing of 1D file transfer and backup 2D homonuclear + 2D processing 2D heteronuclear
Program short intro the hardware locking and shimming 1D proton setup presaturation spectra 13 C spectra, DEPT processing of 1D file transfer and backup 2D homonuclear + 2D processing 2D heteronuclear
Chem 203 December 15, Final Exam Part II Problem 2 of 3 (30 points)
 Name: Chem 203 December 15, 2012 Final Exam Part II Problem 2 of 3 (30 points) Select and submit TWO OUT OF THE THREE PROBLEMS FROM PART II for grading. Do not submit three problems. If you wish to unstaple
Name: Chem 203 December 15, 2012 Final Exam Part II Problem 2 of 3 (30 points) Select and submit TWO OUT OF THE THREE PROBLEMS FROM PART II for grading. Do not submit three problems. If you wish to unstaple
Notes on Experiment #1
 Notes on Experiment #1 Bring graph paper (cm cm is best) From this week on, be sure to print a copy of each experiment and bring it with you to lab. There will not be any experiment copies available in
Notes on Experiment #1 Bring graph paper (cm cm is best) From this week on, be sure to print a copy of each experiment and bring it with you to lab. There will not be any experiment copies available in
Introduction to Oscilloscopes Instructor s Guide
 Introduction to Oscilloscopes A collection of lab exercises to introduce you to the basic controls of a digital oscilloscope in order to make common electronic measurements. Revision 1.0 Page 1 of 25 Copyright
Introduction to Oscilloscopes A collection of lab exercises to introduce you to the basic controls of a digital oscilloscope in order to make common electronic measurements. Revision 1.0 Page 1 of 25 Copyright
The Discussion of this exercise covers the following points:
 Exercise 3-2 Frequency-Modulated CW Radar EXERCISE OBJECTIVE When you have completed this exercise, you will be familiar with FM ranging using frequency-modulated continuous-wave (FM-CW) radar. DISCUSSION
Exercise 3-2 Frequency-Modulated CW Radar EXERCISE OBJECTIVE When you have completed this exercise, you will be familiar with FM ranging using frequency-modulated continuous-wave (FM-CW) radar. DISCUSSION
Bruker BioSpin. Solid State NMR. AVANCE Solids User Manual. Version. NMR Spectroscopy. think forward
 Bruker BioSpin Solid State NMR AVANCE Solids User Manual Version 002 think forward NMR Spectroscopy The information in this manual may be altered without notice. BRUKER BIOSPIN accepts no responsibility
Bruker BioSpin Solid State NMR AVANCE Solids User Manual Version 002 think forward NMR Spectroscopy The information in this manual may be altered without notice. BRUKER BIOSPIN accepts no responsibility
Measurement Procedure & Test Equipment Used
 Measurement Procedure & Test Equipment Used Except where otherwise stated, all measurements are made following the Electronic Industries Association (EIA) Minimum Standard for Portable/Personal Land Mobile
Measurement Procedure & Test Equipment Used Except where otherwise stated, all measurements are made following the Electronic Industries Association (EIA) Minimum Standard for Portable/Personal Land Mobile
DC and AC Circuits. Objective. Theory. 1. Direct Current (DC) R-C Circuit
 [International Campus Lab] Objective Determine the behavior of resistors, capacitors, and inductors in DC and AC circuits. Theory ----------------------------- Reference -------------------------- Young
[International Campus Lab] Objective Determine the behavior of resistors, capacitors, and inductors in DC and AC circuits. Theory ----------------------------- Reference -------------------------- Young
ECE 4670 Spring 2014 Lab 1 Linear System Characteristics
 ECE 4670 Spring 2014 Lab 1 Linear System Characteristics 1 Linear System Characteristics The first part of this experiment will serve as an introduction to the use of the spectrum analyzer in making absolute
ECE 4670 Spring 2014 Lab 1 Linear System Characteristics 1 Linear System Characteristics The first part of this experiment will serve as an introduction to the use of the spectrum analyzer in making absolute
THE INSTRUMENT. I. Introduction
 THE INSTRUMENT I. Introduction Teach Spin's PS1-A is the first pulsed nuclear magnetic resonance spectrometer signed specifically for teaching. It provides physics, chemistry, biology, geology, and other
THE INSTRUMENT I. Introduction Teach Spin's PS1-A is the first pulsed nuclear magnetic resonance spectrometer signed specifically for teaching. It provides physics, chemistry, biology, geology, and other
Laboratory 3 (drawn from lab text by Alciatore)
 Laboratory 3 (drawn from lab text by Alciatore) The Oscilloscope Required Components: 1 10 resistor 2 100 resistors 2 lk resistors 1 2k resistor 2 4.7M resistors 1 0.F capacitor 1 0.1 F capacitor 1 1.0uF
Laboratory 3 (drawn from lab text by Alciatore) The Oscilloscope Required Components: 1 10 resistor 2 100 resistors 2 lk resistors 1 2k resistor 2 4.7M resistors 1 0.F capacitor 1 0.1 F capacitor 1 1.0uF
Instruction manual for T3DS software. Tool for THz Time-Domain Spectroscopy. Release 4.0
 Instruction manual for T3DS software Release 4.0 Table of contents 0. Setup... 3 1. Start-up... 5 2. Input parameters and delay line control... 6 3. Slow scan measurement... 8 4. Fast scan measurement...
Instruction manual for T3DS software Release 4.0 Table of contents 0. Setup... 3 1. Start-up... 5 2. Input parameters and delay line control... 6 3. Slow scan measurement... 8 4. Fast scan measurement...
MT Gemcitabine, [5-3 H(N)]- Lot A NK
![MT Gemcitabine, [5-3 H(N)]- Lot A NK MT Gemcitabine, [5-3 H(N)]- Lot A NK](/thumbs/93/112954466.jpg) MT-1572 Gemcitabine, [5-3 H(N)]- Lot 194-14-234-A-2514-NK A) All chromatograms were run using the HPLC method described on the Product Data Sheet. Concentrations and volumes: Standard solution concentration
MT-1572 Gemcitabine, [5-3 H(N)]- Lot 194-14-234-A-2514-NK A) All chromatograms were run using the HPLC method described on the Product Data Sheet. Concentrations and volumes: Standard solution concentration
The Amazing MFJ 269 Author Jack Tiley AD7FO
 The Amazing MFJ 269 Author Jack Tiley AD7FO ARRL Certified Emcomm and license class Instructor, Volunteer Examiner, EWA Technical Coordinator and President of the Inland Empire VHF Club What Can be Measured?
The Amazing MFJ 269 Author Jack Tiley AD7FO ARRL Certified Emcomm and license class Instructor, Volunteer Examiner, EWA Technical Coordinator and President of the Inland Empire VHF Club What Can be Measured?
Using Frequency Diversity to Improve Measurement Speed Roger Dygert MI Technologies, 1125 Satellite Blvd., Suite 100 Suwanee, GA 30024
 Using Frequency Diversity to Improve Measurement Speed Roger Dygert MI Technologies, 1125 Satellite Blvd., Suite 1 Suwanee, GA 324 ABSTRACT Conventional antenna measurement systems use a multiplexer or
Using Frequency Diversity to Improve Measurement Speed Roger Dygert MI Technologies, 1125 Satellite Blvd., Suite 1 Suwanee, GA 324 ABSTRACT Conventional antenna measurement systems use a multiplexer or
Physics 120 Lab 1 (2018) - Instruments and DC Circuits
 Physics 120 Lab 1 (2018) - Instruments and DC Circuits Welcome to the first laboratory exercise in Physics 120. Your state-of-the art equipment includes: Digital oscilloscope w/usb output for SCREENSHOTS.
Physics 120 Lab 1 (2018) - Instruments and DC Circuits Welcome to the first laboratory exercise in Physics 120. Your state-of-the art equipment includes: Digital oscilloscope w/usb output for SCREENSHOTS.
LLS - Introduction to Equipment
 Published on Advanced Lab (http://experimentationlab.berkeley.edu) Home > LLS - Introduction to Equipment LLS - Introduction to Equipment All pages in this lab 1. Low Light Signal Measurements [1] 2. Introduction
Published on Advanced Lab (http://experimentationlab.berkeley.edu) Home > LLS - Introduction to Equipment LLS - Introduction to Equipment All pages in this lab 1. Low Light Signal Measurements [1] 2. Introduction
MG3740A Analog Signal Generator. 100 khz to 2.7 GHz 100 khz to 4.0 GHz 100 khz to 6.0 GHz
 Data Sheet MG3740A Analog Signal Generator 100 khz to 2.7 GHz 100 khz to 4.0 GHz 100 khz to 6.0 GHz Contents Definitions, Conditions of Specifications... 3 Frequency... 4 Output Level... 5 ATT Hold...
Data Sheet MG3740A Analog Signal Generator 100 khz to 2.7 GHz 100 khz to 4.0 GHz 100 khz to 6.0 GHz Contents Definitions, Conditions of Specifications... 3 Frequency... 4 Output Level... 5 ATT Hold...
Combinational logic: Breadboard adders
 ! ENEE 245: Digital Circuits & Systems Lab Lab 1 Combinational logic: Breadboard adders ENEE 245: Digital Circuits and Systems Laboratory Lab 1 Objectives The objectives of this laboratory are the following:
! ENEE 245: Digital Circuits & Systems Lab Lab 1 Combinational logic: Breadboard adders ENEE 245: Digital Circuits and Systems Laboratory Lab 1 Objectives The objectives of this laboratory are the following:
SpinCore RadioProcessor LabVIEW Extensions
 NMR Interface User's Manual SpinCore Technologies, Inc. http:// Congratulations and thank you for choosing a design from SpinCore Technologies, Inc. We appreciate your business! At SpinCore we try to fully
NMR Interface User's Manual SpinCore Technologies, Inc. http:// Congratulations and thank you for choosing a design from SpinCore Technologies, Inc. We appreciate your business! At SpinCore we try to fully
Workshop on Rapid Scan EPR. University of Denver EPR Center and Bruker BioSpin July 28, 2013
 Workshop on Rapid Scan EPR University of Denver EPR Center and Bruker BioSpin July 28, 2013 Direct detection Direct detected magnetic resonance that is, without modulation and phase-sensitive detection
Workshop on Rapid Scan EPR University of Denver EPR Center and Bruker BioSpin July 28, 2013 Direct detection Direct detected magnetic resonance that is, without modulation and phase-sensitive detection
WE-525T Antenna Analyzer Manual and Specification
 WE-525T Antenna Analyzer Manual and Specification 1.0 Description This product is designed to speed and ease the testing and tuning of antenna systems. Graphical displays of SWR, Return loss, Distance
WE-525T Antenna Analyzer Manual and Specification 1.0 Description This product is designed to speed and ease the testing and tuning of antenna systems. Graphical displays of SWR, Return loss, Distance
If the magnetic field is larger, more energy is required to excite a given nucleus.
 1 2 If an NMR-active nucleus such as 1 H or 13 C is put into a magnet field, then it will come into resonance if it is irradiated with rf at the correct frequency. The correct frequency depends mainly
1 2 If an NMR-active nucleus such as 1 H or 13 C is put into a magnet field, then it will come into resonance if it is irradiated with rf at the correct frequency. The correct frequency depends mainly
CLIP-HSQMBC: Easy measurement of small proton-carbon coupling constants in organic molecules
 CLIP-HSQMBC: Easy measurement of small proton-carbon coupling constants in organic molecules Josep Saurí, a Teodor Parella a and Juan F. Espinosa* b Supporting information 1. Pulse sequence code (Bruker)
CLIP-HSQMBC: Easy measurement of small proton-carbon coupling constants in organic molecules Josep Saurí, a Teodor Parella a and Juan F. Espinosa* b Supporting information 1. Pulse sequence code (Bruker)
781/ /
 781/329-47 781/461-3113 SPECIFICATIONS DC SPECIFICATIONS J Parameter Min Typ Max Units SAMPLING CHARACTERISTICS Acquisition Time 5 V Step to.1% 25 375 ns 5 V Step to.1% 2 35 ns Small Signal Bandwidth 15
781/329-47 781/461-3113 SPECIFICATIONS DC SPECIFICATIONS J Parameter Min Typ Max Units SAMPLING CHARACTERISTICS Acquisition Time 5 V Step to.1% 25 375 ns 5 V Step to.1% 2 35 ns Small Signal Bandwidth 15
Laboratory Exercise 6 THE OSCILLOSCOPE
 Introduction Laboratory Exercise 6 THE OSCILLOSCOPE The aim of this exercise is to introduce you to the oscilloscope (often just called a scope), the most versatile and ubiquitous laboratory measuring
Introduction Laboratory Exercise 6 THE OSCILLOSCOPE The aim of this exercise is to introduce you to the oscilloscope (often just called a scope), the most versatile and ubiquitous laboratory measuring
A Conceptual Tour of Pulsed NMR*
 A Conceptual Tour of Pulsed NMR* Many nuclei, but not all, possess both a magnetic moment, µ, and an angular momentum, L. Such particles are said to have spin. When the angular momentum and magnetic moment
A Conceptual Tour of Pulsed NMR* Many nuclei, but not all, possess both a magnetic moment, µ, and an angular momentum, L. Such particles are said to have spin. When the angular momentum and magnetic moment
100 Hz to 22. HP 8566B Spectrum Analyzer. Discontinued Product Support Information Only. Outstanding Precision and Capability
 Discontinued Product Support Information Only This literature was published years prior to the establishment of Agilent Technologies as a company independent from Hewlett-Packard and describes products
Discontinued Product Support Information Only This literature was published years prior to the establishment of Agilent Technologies as a company independent from Hewlett-Packard and describes products
Getting Started. MSO/DPO Series Oscilloscopes. Basic Concepts
 Getting Started MSO/DPO Series Oscilloscopes Basic Concepts 001-1523-00 Getting Started 1.1 Getting Started What is an oscilloscope? An oscilloscope is a device that draws a graph of an electrical signal.
Getting Started MSO/DPO Series Oscilloscopes Basic Concepts 001-1523-00 Getting Started 1.1 Getting Started What is an oscilloscope? An oscilloscope is a device that draws a graph of an electrical signal.
SERVOSTAR S- and CD-series Sine Encoder Feedback
 SERVOSTAR S- and CD-series Sine Encoder Feedback The SERVOSTAR S and SERVOSTAR CD family of drives offers the ability to accept signals from various feedback devices. Sine Encoders provide analog-encoded
SERVOSTAR S- and CD-series Sine Encoder Feedback The SERVOSTAR S and SERVOSTAR CD family of drives offers the ability to accept signals from various feedback devices. Sine Encoders provide analog-encoded
University of New Hampshire InterOperability Laboratory Gigabit Ethernet Consortium
 University of New Hampshire InterOperability Laboratory Gigabit Ethernet Consortium As of June 18 th, 2003 the Gigabit Ethernet Consortium Clause 40 Physical Medium Attachment Conformance Test Suite Version
University of New Hampshire InterOperability Laboratory Gigabit Ethernet Consortium As of June 18 th, 2003 the Gigabit Ethernet Consortium Clause 40 Physical Medium Attachment Conformance Test Suite Version
The Agilent OneNMR Probe
 The Agilent OneNMR Probe Technical Overview Introduction The Agilent OneNMR probe represents a new class of NMR probes. This technology is the most signifi cant advance in solution-state probes in over
The Agilent OneNMR Probe Technical Overview Introduction The Agilent OneNMR probe represents a new class of NMR probes. This technology is the most signifi cant advance in solution-state probes in over
FlexDDS-NG DUAL. Dual-Channel 400 MHz Agile Waveform Generator
 FlexDDS-NG DUAL Dual-Channel 400 MHz Agile Waveform Generator Excellent signal quality Rapid parameter changes Phase-continuous sweeps High speed analog modulation Wieserlabs UG www.wieserlabs.com FlexDDS-NG
FlexDDS-NG DUAL Dual-Channel 400 MHz Agile Waveform Generator Excellent signal quality Rapid parameter changes Phase-continuous sweeps High speed analog modulation Wieserlabs UG www.wieserlabs.com FlexDDS-NG
TRANSMITTER MODEL: KAS-2030M
 Page 1 of 16 FCC PART 15, SUBPART B and C TEST REPORT for TRANSMITTER MODEL: KAS-2030M Prepared for WILDLIFE TECHNOLOGIES 115 WOLCOTT STREET MANCHESTER, NEW HAMPSHIRE 03103 Prepared by: KYLE FUJIMOTO Approved
Page 1 of 16 FCC PART 15, SUBPART B and C TEST REPORT for TRANSMITTER MODEL: KAS-2030M Prepared for WILDLIFE TECHNOLOGIES 115 WOLCOTT STREET MANCHESTER, NEW HAMPSHIRE 03103 Prepared by: KYLE FUJIMOTO Approved
Optical Pumping Control Unit
 (Advanced) Experimental Physics V85.0112/G85.2075 Optical Pumping Control Unit Fall, 2012 10/16/2012 Introduction This document is gives an overview of the optical pumping control unit. Magnetic Fields
(Advanced) Experimental Physics V85.0112/G85.2075 Optical Pumping Control Unit Fall, 2012 10/16/2012 Introduction This document is gives an overview of the optical pumping control unit. Magnetic Fields
Transfer Function (TRF)
 (TRF) Module of the KLIPPEL R&D SYSTEM S7 FEATURES Combines linear and nonlinear measurements Provides impulse response and energy-time curve (ETC) Measures linear transfer function and harmonic distortions
(TRF) Module of the KLIPPEL R&D SYSTEM S7 FEATURES Combines linear and nonlinear measurements Provides impulse response and energy-time curve (ETC) Measures linear transfer function and harmonic distortions
Eddy current flaw detector «Eddycon C»
 ULTRACON-SERVICE LLC Eddy current flaw detector «Eddycon C» Quick start guide CONTENTS P. 1 CONTROLLERS OF EDDYCON C FLAW DETECTOR... 3 2 SETTINGS OF «TEST» MENU... 5 3 INSTRUCTIONS FOR USE... 8 3.1 THRESHOLD
ULTRACON-SERVICE LLC Eddy current flaw detector «Eddycon C» Quick start guide CONTENTS P. 1 CONTROLLERS OF EDDYCON C FLAW DETECTOR... 3 2 SETTINGS OF «TEST» MENU... 5 3 INSTRUCTIONS FOR USE... 8 3.1 THRESHOLD
IEC Electrical fast transient / Burst immunity test
 CONDUCTED RF EQUIPMENT POWER AMPLIFIERS IEC 61000-4-4 Electrical fast transient / Burst immunity test IEC 61000-4-4 Electrical fast transient / Burst immunity test Markus Fuhrer Phenomenom open a contact
CONDUCTED RF EQUIPMENT POWER AMPLIFIERS IEC 61000-4-4 Electrical fast transient / Burst immunity test IEC 61000-4-4 Electrical fast transient / Burst immunity test Markus Fuhrer Phenomenom open a contact
Filters And Waveform Shaping
 Physics 3330 Experiment #3 Fall 2001 Purpose Filters And Waveform Shaping The aim of this experiment is to study the frequency filtering properties of passive (R, C, and L) circuits for sine waves, and
Physics 3330 Experiment #3 Fall 2001 Purpose Filters And Waveform Shaping The aim of this experiment is to study the frequency filtering properties of passive (R, C, and L) circuits for sine waves, and
HD Radio FM Transmission. System Specifications
 HD Radio FM Transmission System Specifications Rev. G December 14, 2016 SY_SSS_1026s TRADEMARKS HD Radio and the HD, HD Radio, and Arc logos are proprietary trademarks of ibiquity Digital Corporation.
HD Radio FM Transmission System Specifications Rev. G December 14, 2016 SY_SSS_1026s TRADEMARKS HD Radio and the HD, HD Radio, and Arc logos are proprietary trademarks of ibiquity Digital Corporation.
UNIT 2. Q.1) Describe the functioning of standard signal generator. Ans. Electronic Measurements & Instrumentation
 UNIT 2 Q.1) Describe the functioning of standard signal generator Ans. STANDARD SIGNAL GENERATOR A standard signal generator produces known and controllable voltages. It is used as power source for the
UNIT 2 Q.1) Describe the functioning of standard signal generator Ans. STANDARD SIGNAL GENERATOR A standard signal generator produces known and controllable voltages. It is used as power source for the
TopSpin Guide Book. Basic NMR Experiments User Manual. Innovation with Integrity. Version 002 NMR
 TopSpin Guide Book Basic NMR Experiments User Manual Version 002 Innovation with Integrity NMR Copyright by Bruker Corporation All rights reserved. No part of this publication may be reproduced, stored
TopSpin Guide Book Basic NMR Experiments User Manual Version 002 Innovation with Integrity NMR Copyright by Bruker Corporation All rights reserved. No part of this publication may be reproduced, stored
Model 3102D 0-2 kv H.V. Power Supply
 Features Compact single width NIM package Regulated up to ±2000 V dc. 1 ma output Noise and ripple 3 mv peak to peak Overload and short circuit protected Overload, inhibit and polarity status indicators
Features Compact single width NIM package Regulated up to ±2000 V dc. 1 ma output Noise and ripple 3 mv peak to peak Overload and short circuit protected Overload, inhibit and polarity status indicators
A NEW GENERATION PROGRAMMABLE PHASE/AMPLITUDE MEASUREMENT RECEIVER
 GENERAL A NEW GENERATION PROGRAMMABLE PHASE/AMPLITUDE MEASUREMENT RECEIVER by Charles H. Currie Scientific-Atlanta, Inc. 3845 Pleasantdale Road Atlanta, Georgia 30340 A new generation programmable, phase-amplitude
GENERAL A NEW GENERATION PROGRAMMABLE PHASE/AMPLITUDE MEASUREMENT RECEIVER by Charles H. Currie Scientific-Atlanta, Inc. 3845 Pleasantdale Road Atlanta, Georgia 30340 A new generation programmable, phase-amplitude
: REMOTE CONTROL TRANSMITTER : FEGO PRECISION INDUSTRIAL CO., LTD.
 Product : REMOTE CONTROL TRANSMITTER Manufacture : FEGO PRECISION INDUSTRIAL CO., LTD. FCC ID : M8CRL202 Model : BC4162D Report No. : MLT0406P15001 Test Date : 06/07/2004 Test By Max Light Technology Co.,Ltd.
Product : REMOTE CONTROL TRANSMITTER Manufacture : FEGO PRECISION INDUSTRIAL CO., LTD. FCC ID : M8CRL202 Model : BC4162D Report No. : MLT0406P15001 Test Date : 06/07/2004 Test By Max Light Technology Co.,Ltd.
Current Probes. User Manual
 Current Probes User Manual ETS-Lindgren Inc. reserves the right to make changes to any product described herein in order to improve function, design, or for any other reason. Nothing contained herein shall
Current Probes User Manual ETS-Lindgren Inc. reserves the right to make changes to any product described herein in order to improve function, design, or for any other reason. Nothing contained herein shall
ACTR Features: ACTR DCC6C v1.1
 Features: ACTR9028-934.6-DCC6C v1.1 1-port Resonator Provides reliable, fundamental mode, quartz frequency stabilization i.e. in transmitters or local oscillators Surface Mounted Technology (SMT) Lead-free
Features: ACTR9028-934.6-DCC6C v1.1 1-port Resonator Provides reliable, fundamental mode, quartz frequency stabilization i.e. in transmitters or local oscillators Surface Mounted Technology (SMT) Lead-free
Exercise at the Spectrometer
 Exercise at the Spectrometer General The script is written for Bruker spectrometers. The color code means, red for all commands and green for parameters in the TopSpin commando line. The script gives additional
Exercise at the Spectrometer General The script is written for Bruker spectrometers. The color code means, red for all commands and green for parameters in the TopSpin commando line. The script gives additional
Background (~EE369B)
 Background (~EE369B) Magnetic Resonance Imaging D. Nishimura Overview of NMR Hardware Image formation and k-space Excitation k-space Signals and contrast Signal-to-Noise Ratio (SNR) Pulse Sequences 13
Background (~EE369B) Magnetic Resonance Imaging D. Nishimura Overview of NMR Hardware Image formation and k-space Excitation k-space Signals and contrast Signal-to-Noise Ratio (SNR) Pulse Sequences 13
