MINNOVATM INSTRUCTION MANUAL
|
|
|
- Nora Hunter
- 6 years ago
- Views:
Transcription
1 MINNOVATM P E L V I C F L O O R S T I M U L A T I O N S Y S T E M INSTRUCTION MANUAL
2 Table of Contents Introduction... 3 Product Description... 3 Indications for Use Contraindications Warnings Precautions Before Treatment Minnova Features Initial Fitting and Treatment... 8 Typical Treatment Protocols... 9 Caring For The Device...10 Technical Information Electromagnetic Compatibility (EMC) Tables...13 Description of Device Markings...17 Limited Warranty and Disclaimer CAUTION: Federal law (USA) restricts this device to sale by, or on the order of, a physician. 2
3 Introduction The Minnova TM Pelvic Floor Stimulation (PFS) system uses mild electrical stimulation to help control or cure urinary incontinence. The system consists of an external, batterypowered stimulator and an electrode. Product Description Minnova system components and accessories include: Minnova pelvic floor stimulator ComfortPulse vaginal or rectal electrode Two AA (1.5V) alkaline batteries Carrying case Instruction manual Indications for Use Pelvic Floor Stimulation is indicated for acute and ongoing treatment of urinary incontinence in cases where the following results may improve urinary control: Improvement of urethral sphincter closure Strengthening of pelvic floor muscles Inhibition of the detrusor muscle through reflexive mechanisms 3
4 ! Contraindications Minnova should not be used if the patient has any of the following conditions: Active urinary tract infection Pregnancy or attempting pregnancy Recent history of vaginal bleeding between menstrual periods Infections or lesions in the area of electrode placement Diminished sensory perception Inability to understand the directions for use or operate the device correctly History of urinary retention or post-void residual volume greater than 200cc History of cardiac arrhythmia! Demand type implanted pacemaker or defibrillator Warnings Review the following warnings before using Minnova: The patient should discontinue use and immediately consult a clinician if irritation, pain or unusual bleeding occurs. Keep out of reach of children. Do not use in water (for example, while bathing). This equipment is not rated for use while immersed or under dripping water. Do not use simultaneously with high frequency hospital diagnostic/therapeutic equipment. Doing so may result in burns at the site of the electrodes and possible damage to the device. Do not use simultaneously with patient monitoring equipment such as EKG monitors without investigating the possibility of output from the device causing erroneous monitor readings. 4
5 Do not attempt to plug the electrode lead wire into wall sockets or line cord receptacles (i.e. extension cords) under any circumstances. Doing so could result in severe shock or burns. Do not carry batteries in a pocket, purse, or any other place where the terminals could become short circuited, (e.g. by way of a coin or paper clip). Intense heat could be generated and injury may result. The long term effects of chronic electrical stimulation are unknown. Do not use with products other than those recommended by Empi. 5
6 ! Precautions Review the following precautions: It is not recommended to use device while under the influence of alcohol or other substances, such as sleeping pills or tranquilizers, which could affect the patient s ability to operate the system correctly. Removing or inserting the electrode while the system is on could result in discomfort. Use while operating hazardous equipment, machinery or motorized vehicles could cause abrupt changes in stimulation which can startle the patient and create a hazard. Do not use in close proximity (e.g. <1m) to transmitting cellular (wireless) telephones or two-way radios. This equipment may produce instability in the stimulator output. Sudden unexpected changes in output could startle the patient and create a hazard. Use while sleeping is not recommended. The patient should discontinue use and consult a clinician if a significant change in the stimulation sensation occurs without changing the intensity settings. It is normal to feel a difference in sensation when changing positions during stimulation. Do not use in close proximity such as 3 feet (1meter) to shortwave or microwave therapy equipment. This equipment may produce instability in the stimulator output or may shut the stimulator off. Sudden unexpected changes in output could startle the patient and create a hazard. 6
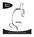
7 Before Treatment An examination of the rectal or vaginal tissue should be completed prior to initial treatment to rule out any contraindications, establish the baseline tissue condition and assess the physiology discussed below. Pelvic floor stimulation may not be successful if the urethra has excessive scarring or if there is significant denervation of the pelvic floor. The neuromuscular structures involved in urine storage must also be at least partially preserved. Patients with intact sacral reflex arcs and some innervation of the pelvic floor may benefit from pelvic floor stimulation. Minnova Features The Minnova device features are described below and shown in Figure Intensity Control Knob controls the intensity level. Note: The numerical scale is for reference only. It is not intended to correlate to any measurable output parameter of the device. 2. Electrode Connector connects the electrode lead wire to the device. Figure Frequency Selection Slide Switch selects the stimulation frequency of either 50Hz or 12.5Hz. 4. Session Start Indicator Light blinks rapidly for 10 seconds when Start button is pressed. 5. START Button initiates the 15 minute intermittent stimulation of 5 seconds of stimulation followed by 10 seconds of rest. 6. Battery Compartment secures two AA (1.5V) batteries. Note: A Ni-Cd rechargeable battery is not recommended because it does not provide the device with enough power, and the device may shut off after a very short time. 7. Stimulation Indicator Light illuminates when the Intensity Control Knob is turned on and during the 5 second stimulation phase. 7
8 Initial Fitting and Treatment The initial fitting of the electrode and the initial treatment session should be done under medical supervision. 1. Before inserting the electrode, make certain that the electrode lead wire is not connected to the device. 2. Insert and position the electrode per the electrode instructions for use. 3. Do not turn the device on unless the electrode is positioned properly. 4. Verify that the device is OFF. To turn the device off, the intensity control knob should be turned to 0 so that you hear a click. The click indicates that the device is off. 5. Connect the electrode lead wire to the device. 6. Set the frequency selection to the appropriate frequency, 12.5Hz or 50Hz. 7. Turn the device ON and set the stimulation intensity level by slowly turning the Intensity Control Knob to the most comfortable level which elicits a pelvic floor contraction. If there is discomfort, turn the intensity down to a comfortable level and check that the electrode is positioned properly. 8. Once desired intensity is set, press the START button to initiate a 15 minute treatment session. 9. After treatment session is completed, turn the device OFF by turning the intensity control knob to the 0 position. Note: The click indicates that the device is off. 10. Disconnect the electrode from the device before removing the electrode. 11. Remove the electrode and clean by following the Electrode Cleaning Instructions. 8
9 Typical PFS Treatment Protocols The treatment protocol prescribed will vary depending upon the type of incontinence being treated. The following treatment protocols are recommended based upon current clinical studies. 1 Protocol for Genuine Stress Incontinence (GSI): Frequency: 50Hz Use: Twice a day (once in morning, once in evening), daily or every other day Session Length: 15 minutes Protocol for Urge Incontinence: Frequency: 12.5Hz Use: Twice a day (once in morning, once in evening), daily or every other day Session Length: 15 minutes Protocol for Mixed Incontinence (Combination of Stress and Urge Incontinence): Frequencies: 50Hz and 12.5Hz Use: Use 50Hz in the morning, and 12.5Hz in the evening, daily or every other day Session Length: 15 minutes Treatment Duration For maximum benefit, the Minnova Pelvic Floor Stimulation System should be used according to prescribed protocols for approximately 20 weeks. Maintenance In order to maintain the degree of continence achieved during the first 20 weeks of treatment, the patient should continue to use the device 2-3 times a week or as needed for their level of dryness. 1 Richardson D., Miller K., Siegel S., Karram M. Pelvic Floor Electrical Stimulation: A Comparison of Daily to Every Other Day Therapy for Genuine Stress Incontinence. Urology 48: ,
10 Caring For The Device Cleaning To clean the Minnova device, wipe it with a cloth moistened with soap and water. Never immerse the device in water or other liquids. Maintenance Under normal conditions, the device does not require periodic preventative maintenance or calibration; however, all information necessary to perform periodic safety checks by a qualified technician is included in the Technical Information Section. Changing the Battery Change the battery when indicated by a SLOWLY blinking green light next to the start button. Be sure polarity ( + and ) markings on battery match with markings on device. Repair There are no user serviceable parts inside the device. If the device becomes damaged or needs repair, please contact the Clear Lake Service Center at for instructions. In the case of repairs or returns outside of North America, contact your Authorized Empi Distributor, or contact Empi directly. Storage To properly store the device for an extended period of time, i.e. 90 days or more, remove the battery from the device and store the device in a dry location. Disposal To properly dispose of the device, ship the device, postage prepaid, to Clear Lake Service Center. Please enclose a note indicating that the item is being returned for disposal or recycling. 10
11 Technical Information Standard Conditions (Unless otherwise indicated.) 2 x 1.5V battery voltage, 500Ω resistive load, 23 C Output Specifications (Tolerances ± 15% unless noted) Output type Waveform Amplitude Range Amplitude regulated current source over the range of 100Ω to 500Ω. Alternating (balanced symmetrical biphasic with no dc component, See figure A) 0 to 100mA Pulse width at 50% amplitude 300µs Pulse frequency Maximum rms current 12.5Hz or 50Hz 20mA Maximum charge per pulse 60µC Duty cycle Session duration Battery Information ON time: 5 seconds OFF time: 10 seconds 15 minutes Expected typical battery life at 40mA intensity >30h Recommended battery Required Electrodes Two AA 1.5V DC alkaline, IEC R6, such as Eveready No. 91. Do not use a Ni-Cd rechargeable battery or device may shut down after a short period of time. Empi electrodes with custom touchproof connector. 11
12 IEC 601 Classification Type BF applied part. Class II device. Internally powered only. Ordinary protection against ingress of liquids. Continuous operation. Not suitable for use in the presence of a flammable anaesthetic mixture with air or with oxygen or nitrous oxide. Physical Dimensions Approximate Size (H x W x D) Approximate weight (without battery) Environmental Conditions 110mm x 70mm x 30mm (4.3 in x 2.8 in x 1.2 in) 100 grams (4 oz) Operation 10 C to 50 C (+50 F to +122 F); Up to 75% relative humidity (non-condensing); Air pressure 50kPa to 106kPa. Transportation & storage Store in a cool dry place -40 C to +70 C (-40 F to +158 F); 10% to 100% relative humidity condensing; Air pressure 50kPa to 106kPa Waveform Shown is Typical Balanced symmetrical biphasic waveform. Output current into 500Ω, 750Ω and 1kΩ resistive load at maximum intensity setting. current amplitude [ma] Simulated Minnova output waveform in the time domain up to 500 Ohm load 750 Ohm load 1 kohm load time [µs] Figure A. 12
13 Electromagnetic Compatibility (EMC) Tables Guidance and manufacturer s declaration electromagnetic emissions The Minnova is intended for use in the electromagnetic environment specified below. The customer or the user of the Minnova should assure that it is used in such an environment. RF emissions CISPR 11 RF emissions CISPR 11 Emission tests Harmonic emissions IEC Voltage fluctuations/ flicker emissions IEC Group 1 Class B Compliance Not Applicable- Battery Operated Device Not Applicable- Battery Operated Device Electromagnetic environment - guidance The Minnova uses RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment. The Minnova is suitable for use in all establishments, including domestic establishments and those directly connected to the public low-voltage power supply network that supplies buildings used for domestic purposes. 13
14 Electromagnetic Compatibility (EMC) Tables Guidance and manufacturer s declaration electromagnetic immunity The Minnova is intended for use in the electromagnetic environment specified below. The customer or the user of the Minnova should assure that it is used in such an environment. Immunity test IEC test level Compliance level Electromagnetic environment - guidance Electrostatic discharge (ESD) IEC ±6kV contact ±8kV air ±6kV contact ±8kV air Floors should be wood, concrete or ceramic tile. If floors are covered with synthetic material, the relative humidity should be at least 30%. Electrical fast transient/burst IEC ±2kV for power supply lines ±1kV for input/ output lines Not Applicable- Battery Operated Device Mains power quality should be that of a typical commercial or hospital environment. Surge IEC ±1kV differential mode ±2kV common mode Not Applicable- Battery Operated Device Mains power quality should be that of a typical commercial or hospital environment. <5% U T (>95% dip in U T ) for 0.5 cycle Voltage dips, short interruptions and voltage variations on power supply input lines IEC % U T (60% dip in U T ) for 5 cycles 70% U T (30% dip in U T ) for 25 cycles Not Applicable- Battery Operated Device Mains power quality should be that of a typical commercial or hospital environment. <5% U T (>95% dip in U T ) for 5 sec Power frequency (50/60Hz) magnetic field IEC A/m 3 A/m Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment. NOTE U T is the a.c mains voltage prior to application of the test level. 14
15 Electromagnetic Compatibility (EMC) Tables Guidance and manufacturer s declaration electromagnetic immunity The Minnova is intended for use in the electromagnetic environment specified below. The customer or the user of the Minnova should assure that it is used in such an environment. Immunity test IEC test level Compliance level Electromagnetic environment - guidance Portable and mobile RF communications equipment should be used no closer to any part of the Minnova, including cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter. Recommended separation distance Conducted RF IEC Vrms 150 khz to 80 MHz 3V d = [3.5] P V1 Radiated RF IEC V/m 80 MHz to 2.5 GHz 3 V/m d = [3.5] P 80 MHz to 800 MHz E 1 d = [7] P 800 MHz to 2.5 GHz E 1 where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer and d is the recommended separation distance in meters (m). Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey a, should be less than the compliance level in each frequency range b. Interference may occur in the vicinity of equipment marked with the following symbol: NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies. NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. a Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the Minnova is used exceeds the applicable RF compliance level above, the Minnova should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as reorienting or relocating the Minnova. b Over the frequency range 150 khz to 80 MHz, field strengths should be less than 3 V/m. 15
16 Electromagnetic Compatibility (EMC) Tables Recommended separation distances between portable and mobile RF communications equipment and the Minnova The Minnova is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the Minnova can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the Minnova as recommended below. Rated maximum output power of transmitter W Separation distance according to frequency of transmitter m 150 khz to 80 MHz d = [3.5] P V1 80 MHz to 800 MHz d = [3.5] P E1 800 MHz to 2.5 GHz d = [7] P E For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer. NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies. NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. 16
17 Description of Device Markings The markings on your Minnova device are your assurance of its conformity to the highest applicable standards of medical equipment safety and electromagnetic compatibility. One or more of the following markings may appear on your device. Council Directive 93/42/EEC Concerning Medical Devices (Medical Device Directive) 0473 Classified by Underwriters Laboratories, Inc with respect to electric shock, fire and mechanical hazards only in accordance with UL , CAN/CSA C22.2 No M90 Medical Electrical Equipment. 11N1 17
18 Limited Warranty and Disclaimer I. Warning While, in the opinion of Empi, Inc. ( Empi ), the use of the Minnova Pelvic Floor Stimulator (the Product ) has met with some success in the treatment of incontinence, Empi makes no warranties to the purchaser as to the effectiveness of the product. II. Warranty A. Empi warrants to the initial Purchaser ( Purchaser ) (and to no other person) that the Product and the components thereof, distributed or manufactured by Empi, shall be free from defects in workmanship and materials for one year after the date of purchase (the Warranty Period ). B. Accessories including, but not limited to the electrode, electrode cleanser and batteries are excluded from the Warranty and are sold As Is because their structure is such that they may be easily damaged before or during use. III. Limitations of Liabilities and Disclaimer of Warranties A. Empi s sole obligation in the case of any breach of the Limited Warranty set forth in Paragraph II(A) above, shall be, at Empi s option, to repair or replace the Product or parts of the Product without charge to Purchaser or to refund the purchase price of the Product. In order to recover under the Limited Warranty, Purchaser must send Empi written notice of the defect (setting forth the problem in reasonable detail) prior to expiration of the applicable Warranty Period, and within 30 days of discovery of the defect. Upon Empi s written request and authorization, Purchaser shall return the Product to Empi, freight and insurance prepaid, for inspection. Notice and return shipment shall be sent to Empi, Inc., SD Hwy 22, Clear Lake, SD, USA or to an Empi Authorized Service Center. To locate the appropriate Service Center outside of North America, or to request shipment approval, contact Empi directly. Empi will not be responsible for damage due to improper packaging or shipment. If Empi determines in its sole reasonable discretion that the Product contains defective workmanship or materials, Empi will refund to the Purchaser the purchase price for the defective product, or return the repaired Product or a replacement thereof to Purchaser, freight and insurance prepaid, as soon as reasonably possible following receipt of the Product by Empi. If Empi determines in its sole reasonable discretion that the Product does not contain defective workmanship or materials, Empi will return the Product to the Purchaser, freight and insurance billed to the Purchaser. B. This Limited Warranty is voided immediately as to any Product which has been repaired or modified by any person other than authorized employees or agents of Empi or which has been subjected to misuse, abuse, neglect, damage in transit, accident or negligence. C. EXCEPT AS PROVIDED IN PARAGRAPH II(A), THE PRODUCT IS BEING SOLD ON AN AS IS BASIS, ALL ACCESSORIES ARE SOLD AS IS, AND THE ENTIRE RISK AS TO THE QUALITY AND PERFORMANCE OF THE PRODUCT IS WITH PURCHASER. THE LIMITED WARRANTY PROVIDED IN PARAGRAPH II(A) IS INTENDED SOLELY FOR THE BENEFIT OF THE INITIAL PURCHASER AND EMPI DISCLAIMS ALL OTHER WARRANTIES, EXPRESS OR IMPLIED, 18
19 INCLUDING, BUT NOT LIMITED TO, ANY IMPLIED WARRANTIES OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE. PROVIDED, HOWEVER, THAT NOTWITHSTANDING THE FOREGOING SENTENCE, IN THE EVENT AN IMPLIED WARRANTY IS DETERMINED TO EXIST, THE PERIOD FOR PERFORMANCE BY EMPI THEREUNDER SHALL BE LIMITED TO THE LIFETIME OF THE INITIAL PURCHASER. NO EMPLOYEE, REPRESENTATIVE OR AGENT OF EMPI HAS ANY AUTHORITY TO BIND EMPI TO ANY AFFIRMATION, REPRESENTATION OR WARRANTY EXCEPT AS STATED IN THIS WRITTEN LIMITED WARRANTY. (This warranty gives Purchaser specific legal rights and Purchaser may also have other rights which vary from state to state. Some states do not allow limitations of how long an implied warranty lasts, so the above limitation may not apply to the Purchaser.) D. EMPI SHALL NOT BE LIABLE TO ANY PERSON FOR ANY DIRECT, INDIRECT, SPECIAL, INCIDENTAL, OR CONSEQUENTIAL DAMAGES, LOST PROFITS OR MEDICAL EXPENSES CAUSED BY ANY DEFECT, FAILURE, MALFUNCTION OR OTHERWISE OF THE PRODUCT, REGARDLESS OF THE FORM IN WHICH ANY LEGAL OR EQUITABLE ACTION MAY BE BROUGHT AGAINST EMPI (SUCH AS CONTRACT, NEGLIGENCE OR OTHERWISE) THE REMEDY PROVIDED IN PARAGRAPH III(A) ABOVE SHALL CONSTITUTE PURCHASER S SOLE REMEDY. IN NO EVENT SHALL EMPI S LIABILITY UNDER ANY CAUSE OF ACTION RELATING TO THE PRODUCT EXCEED THE PURCHASE PRICE OF THE PRODUCT. (This warranty gives Purchaser specific legal rights and Purchaser may also have other rights which vary from state to state. Some states do not allow limitations of how long an implied warranty lasts, so the above limitation may not apply to the Purchaser.) 19
20 Your authorized representative: (MDSS) Medical Device Safety Service GmbH Schiffgraben 41, Hannover, Germany Tel: Empi, Inc. 205 Hwy 22 East, Clear Lake, SD, USA ; Rev. A, 2010 Empi 6/10 U.S. Patent: D319,881; Re 32,091
Powered Traction Unit OPERATION MANUAL
 Powered Traction Unit OPERATION MANUAL CONTENTS Symbols Safety precautions Symbol for CAUTION Symbol for CONSULT INSTRUCTIONS FOR USE Symbol for SERIAL NUMBER Symbol for CATALOGUE NUMBER Symbol for AUTHORISED
Powered Traction Unit OPERATION MANUAL CONTENTS Symbols Safety precautions Symbol for CAUTION Symbol for CONSULT INSTRUCTIONS FOR USE Symbol for SERIAL NUMBER Symbol for CATALOGUE NUMBER Symbol for AUTHORISED
D C 01/2019 3
 D-0117968-C 01/2019 3 4 D-0117968-C 01/2019 Screw Driver Screw Driver Unplug both the Red & Blue connectors. (see above) Place a small flat head screw driver on the small orange tabs and push down while
D-0117968-C 01/2019 3 4 D-0117968-C 01/2019 Screw Driver Screw Driver Unplug both the Red & Blue connectors. (see above) Place a small flat head screw driver on the small orange tabs and push down while
General Safety/EMC and Electrical Information for i-limb ultra and i-limb digits
 1. General Safety 1.1 The i-limb ultra and i-limb digits devices are electrical devices, which under certain circumstances could present an electrical shock hazard to the user. Please read the accompanying
1. General Safety 1.1 The i-limb ultra and i-limb digits devices are electrical devices, which under certain circumstances could present an electrical shock hazard to the user. Please read the accompanying
BIODEX MULTI- JOINT SYSTEM
 BIODEX MULTI- JOINT SYSTEM CONFORMANCE TO STANDARDS 850-000, 840-000, 852-000 FN: 18-139 5/18 Contact information Manufactured by: Biodex Medical Systems, Inc. 20 Ramsey Road, Shirley, New York, 11967-4704
BIODEX MULTI- JOINT SYSTEM CONFORMANCE TO STANDARDS 850-000, 840-000, 852-000 FN: 18-139 5/18 Contact information Manufactured by: Biodex Medical Systems, Inc. 20 Ramsey Road, Shirley, New York, 11967-4704
MedRx Avant Polar HIT AH-I-MPHITS-5 Effective 11/07/11
 INSTALLATION MANUAL 2 Contents Getting To Know Your AVANT POLAR HIT TM... 4 Setting up the System... 6 Software Installation... 7 Driver Installation Windows 7... 10 Driver Installation Windows XP... 13
INSTALLATION MANUAL 2 Contents Getting To Know Your AVANT POLAR HIT TM... 4 Setting up the System... 6 Software Installation... 7 Driver Installation Windows 7... 10 Driver Installation Windows XP... 13
Guidance and Declaration - Electromagnetic Compatibility (EMC) for the Delfi PTS ii Portable Tourniquet System
 Guidance and Declaration - Electromagnetic Compatibility (EMC) for the Delfi TS ii ortable Tourniquet System Guidance and manufacturer s declaration electromagnetic emissions The TS ii ortable Tourniquet
Guidance and Declaration - Electromagnetic Compatibility (EMC) for the Delfi TS ii ortable Tourniquet System Guidance and manufacturer s declaration electromagnetic emissions The TS ii ortable Tourniquet
English
 English Specifications Type Power Source Vibration Frequency Maximum Output Power Consumption Water Pressure Lighting NE134 AC120V 50/60Hz AC230V 50/60Hz 28~32kHz 8W Max. 42VA 0.1~0.5MPa (1~5kgf/cm
English Specifications Type Power Source Vibration Frequency Maximum Output Power Consumption Water Pressure Lighting NE134 AC120V 50/60Hz AC230V 50/60Hz 28~32kHz 8W Max. 42VA 0.1~0.5MPa (1~5kgf/cm
Electromagnetic compatibility Guidance and manufacturer s declaration DIN EN :2007 (IEC :2007)
 Compressor set Equipment Under Test (EUT) Type 028 Type 047 Type 052 Type 085 Electromagnetic compatibility Guidance and manufacturer s declaration DIN EN 60601-1-2:2007 (IEC 60601-1-2:2007) 2017 PARI
Compressor set Equipment Under Test (EUT) Type 028 Type 047 Type 052 Type 085 Electromagnetic compatibility Guidance and manufacturer s declaration DIN EN 60601-1-2:2007 (IEC 60601-1-2:2007) 2017 PARI
HeRO duet
 HeRO duet CUSTOMER SERVICE TABLE OF CONTENTS TABLE OF CONTENTS OVERVIEW OVERVIEW OVERVIEW OVERVIEW USING HeRO duet USING HeRO duet USING HeRO duet Current HeRO Score HeRO USING HeRO duet USING HeRO duet
HeRO duet CUSTOMER SERVICE TABLE OF CONTENTS TABLE OF CONTENTS OVERVIEW OVERVIEW OVERVIEW OVERVIEW USING HeRO duet USING HeRO duet USING HeRO duet Current HeRO Score HeRO USING HeRO duet USING HeRO duet
Rolyan Splint Pan OPERATION MANUAL. Item # Small Item # Large
 Rolyan Splint Pan OPERATION MANUAL Item #081544816 - Small Item #081544808 Large PLEASE READ THIS ENTIRE MANUAL BEFORE OPERATING YOUR NEW SPLINT PAN. Failure to follow these instructions could result in
Rolyan Splint Pan OPERATION MANUAL Item #081544816 - Small Item #081544808 Large PLEASE READ THIS ENTIRE MANUAL BEFORE OPERATING YOUR NEW SPLINT PAN. Failure to follow these instructions could result in
#
 INSTALLATION MANUAL 2 Contents Getting To Know Your AVANT POLAR HIT TM... 4 Setting up the System... 6 Software Installation... 7 Driver Installation Windows 7... 10 Driver Installation Windows XP... 13
INSTALLATION MANUAL 2 Contents Getting To Know Your AVANT POLAR HIT TM... 4 Setting up the System... 6 Software Installation... 7 Driver Installation Windows 7... 10 Driver Installation Windows XP... 13
(c) Medisave UK. Notice. Edition 1. i-pad Operator s Manual
 Edition 1 Notice i-pad Operator s Manual CU Medical Systems, Inc. reserves the right to make changes on the device specifications contained in this manual at any time without prior notice or obligation
Edition 1 Notice i-pad Operator s Manual CU Medical Systems, Inc. reserves the right to make changes on the device specifications contained in this manual at any time without prior notice or obligation
Nursing Beds with Dewert drive system
 Nursing Beds with Dewert drive system GB Casa Med Classic 4 / Classic (FS) Casa Med Ultra / Ultra (FS) Casa Med Classic Low Casa Med Classic (FS) 4 / Classic / Casa FS Med / Casa Classic Med Low Ultra
Nursing Beds with Dewert drive system GB Casa Med Classic 4 / Classic (FS) Casa Med Ultra / Ultra (FS) Casa Med Classic Low Casa Med Classic (FS) 4 / Classic / Casa FS Med / Casa Classic Med Low Ultra
OtoRead - Technical Specifications Page 0. Technical Specifications. OtoRead D A 2017/06
 OtoRead - Technical Specifications Page 0 Technical Specifications OtoRead D-0116698-A 2017/06 OtoRead - Technical Specifications Page 1 OtoRead TM Configuration Overview The OtoReadTM is available in
OtoRead - Technical Specifications Page 0 Technical Specifications OtoRead D-0116698-A 2017/06 OtoRead - Technical Specifications Page 1 OtoRead TM Configuration Overview The OtoReadTM is available in
PHYSIOFLOW Q-LINK TM
 PHYSIOFLOW Q-LINK TM Service Manual Thursday, 20 October 2016 First placing on the market : 18 January 2012 User Manual PhysioFlow Q-Link 1/17 Table of contents 1. General Information... 3 About this manual...
PHYSIOFLOW Q-LINK TM Service Manual Thursday, 20 October 2016 First placing on the market : 18 January 2012 User Manual PhysioFlow Q-Link 1/17 Table of contents 1. General Information... 3 About this manual...
User Instruction Computer Assisted Local Analgesia. 337 Marion, Le Gardeur QC, Canada, J5Z 4W8
 User Instruction Computer Assisted Local Analgesia 1-800-667-9622 337 Marion, Le Gardeur QC, Canada, J5Z 4W8 USER INSTRUCTION Congratulations on your new CALAJECT! Please read these instructions thoroughly
User Instruction Computer Assisted Local Analgesia 1-800-667-9622 337 Marion, Le Gardeur QC, Canada, J5Z 4W8 USER INSTRUCTION Congratulations on your new CALAJECT! Please read these instructions thoroughly
Biological Safety. Electromagnetic Compatibility (EMC) Observe the following precautions related to biological safety.
 Biological Safety Observe the following precautions related to biological safety. WARNING: Non-medical (commercial) grade peripheral monitors have not been verified or validated by SonoSite as being suitable
Biological Safety Observe the following precautions related to biological safety. WARNING: Non-medical (commercial) grade peripheral monitors have not been verified or validated by SonoSite as being suitable
INSTALLATION MANUAL AVANT HIT+ Hearing Instrument Test Chamber.
 INSTALLATION MANUAL AVANT Hearing Instrument Test Chamber HIT+ www.medrx-int.com Contents Getting To Know Your AVANT HIT+. 3 Computer Requirements 4 Setup System. 5 Software Installation.. 6 Sound Card
INSTALLATION MANUAL AVANT Hearing Instrument Test Chamber HIT+ www.medrx-int.com Contents Getting To Know Your AVANT HIT+. 3 Computer Requirements 4 Setup System. 5 Software Installation.. 6 Sound Card
User Manual. Before Using Your WiTouch Pro Device
 User Manual Before Using Your WiTouch Pro Device Sync the Remote Control and the WiTouch Pro Device. Using the provided screwdriver, remove the back cover from the WiTouch Pro device. 2. Remove the clear
User Manual Before Using Your WiTouch Pro Device Sync the Remote Control and the WiTouch Pro Device. Using the provided screwdriver, remove the back cover from the WiTouch Pro device. 2. Remove the clear
Not for print. Microphone Test Device with SONNET MTD Adapter. User Manual. Cochlear Implants NOT FOR PRINT
 Cochlear Implants Microphone Test Device with SONNET MTD Adapter User Manual AW32690_1.0 (English) Table of contents 1. Table of contents 2. INTRODUCTION 3 Product description 3 3. INTENDED USE INDICATIONS
Cochlear Implants Microphone Test Device with SONNET MTD Adapter User Manual AW32690_1.0 (English) Table of contents 1. Table of contents 2. INTRODUCTION 3 Product description 3 3. INTENDED USE INDICATIONS
TH008F Multi-function Infrared Forehead Thermometer
 TH008F Multi-function Infrared Forehead Thermometer Specifications Functions Temperature measurement range: Forehead mode: 34~42.2 C (93.2~108 F), Surface mode: -22~80 C (-7.6~176 F) Operating temperature
TH008F Multi-function Infrared Forehead Thermometer Specifications Functions Temperature measurement range: Forehead mode: 34~42.2 C (93.2~108 F), Surface mode: -22~80 C (-7.6~176 F) Operating temperature
USER MANUAL MHS-2500I. Please take time to read these instructions before starting to use the scale. Version /17
 USER MANUAL MHS-2500I Please take time to read these instructions before starting to use the scale Version 1.0 05/17 Contents Introduction 3 Product Specification 3 Safety Instructions 4 Explanation of
USER MANUAL MHS-2500I Please take time to read these instructions before starting to use the scale Version 1.0 05/17 Contents Introduction 3 Product Specification 3 Safety Instructions 4 Explanation of
INSTRUCTIONS FOR YOUR NEW PLASMAFLOW. Vascular Therapy System (Compressible Limb Sleeve Device)
 INSTRUCTIONS FOR YOUR NEW PLASMAFLOW Vascular Therapy System (Compressible Limb Sleeve Device) Customer Service Toll Free: 888-508-0712 Email: CustomerService@manamed.net Web: www.manamed.net 1511 W. Alton
INSTRUCTIONS FOR YOUR NEW PLASMAFLOW Vascular Therapy System (Compressible Limb Sleeve Device) Customer Service Toll Free: 888-508-0712 Email: CustomerService@manamed.net Web: www.manamed.net 1511 W. Alton
SAVI SCOUT Surgical Guidance System. Console Operation Manual
 SAVI SCOUT Surgical Guidance System Console Operation Manual 2 Copyrights and Trademarks 2016 Cianna Medical, Inc. All rights reserved. Patents pending. Cianna Medical and SAVI are registered trademarks
SAVI SCOUT Surgical Guidance System Console Operation Manual 2 Copyrights and Trademarks 2016 Cianna Medical, Inc. All rights reserved. Patents pending. Cianna Medical and SAVI are registered trademarks
Technical Data. Electrocardiograph ECG-1250K TD.ECG1250_L. This technical data may be revised or replaced by Nihon Kohden at any time without notice.
 Technical Data Electrocardiograph ECG-1250K This technical data may be revised or replaced by Nihon Kohden at any time without notice. TD.ECG1250_L Specifications ECG input Input impedance: 20 MΩ Electrode
Technical Data Electrocardiograph ECG-1250K This technical data may be revised or replaced by Nihon Kohden at any time without notice. TD.ECG1250_L Specifications ECG input Input impedance: 20 MΩ Electrode
INTRODUCTION. 4 SAFETY INSTRUCTIONS. 5 ABOUT THIS DEVICE. 6 FIRST OPERATION. 7 THE DISPLAY. 8 ATTACHING THE WRIST SLEEVE. 9 CORRECT MEASUREMENT.
 Table of contents INTRODUCTION.............................. 4 SAFETY INSTRUCTIONS........................ 5 ABOUT THIS DEVICE.......................... 6 FIRST OPERATION............................ 7
Table of contents INTRODUCTION.............................. 4 SAFETY INSTRUCTIONS........................ 5 ABOUT THIS DEVICE.......................... 6 FIRST OPERATION............................ 7
Technical Specifications Micromedical VisualEyes 505 by Interacoustics
 VisualEyes 505 - Technical Specifications Page 0 Technical Specifications Micromedical VisualEyes 505 by Interacoustics D-0115523-B 2018/02 VisualEyes 505 - Technical Specifications Page 1 Included and
VisualEyes 505 - Technical Specifications Page 0 Technical Specifications Micromedical VisualEyes 505 by Interacoustics D-0115523-B 2018/02 VisualEyes 505 - Technical Specifications Page 1 Included and
#0086.
 INSTALLATION MANUAL Contents Getting to Know Your AVANT REM Speech+... 3 Software Installation... 4 Driver Installation Windows 7... 7 EMC Precautions... 11 Safety... 15 Limited Warranty... 18 #0086 www.medrx-usa.com
INSTALLATION MANUAL Contents Getting to Know Your AVANT REM Speech+... 3 Software Installation... 4 Driver Installation Windows 7... 7 EMC Precautions... 11 Safety... 15 Limited Warranty... 18 #0086 www.medrx-usa.com
: 0089 GTIN
 GTIN: 00894912002050 PRECAUTIONS PROBE Non-invasive probes are for transcutaneous use only Probe transducer tips are thin and delicate. Be careful not to drop or hit the probe tip. After use, protect the
GTIN: 00894912002050 PRECAUTIONS PROBE Non-invasive probes are for transcutaneous use only Probe transducer tips are thin and delicate. Be careful not to drop or hit the probe tip. After use, protect the
Transcutaneous Electrical Nerve Stimulator TENS 212. Instruction Manual. Read before using
 Transcutaneous Electrical Nerve Stimulator TENS 212 Instruction Manual Read before using TABLE OF CONTENTS GENERAL DESCRIPTION 1 SYSTEM COMPONENTS 1 WARRANTY 1 INDICATIONS AND CONTRAINDICATIONS 2 WARNINGS
Transcutaneous Electrical Nerve Stimulator TENS 212 Instruction Manual Read before using TABLE OF CONTENTS GENERAL DESCRIPTION 1 SYSTEM COMPONENTS 1 WARRANTY 1 INDICATIONS AND CONTRAINDICATIONS 2 WARNINGS
Controller - Instructions For Use
 Controller - Instructions For Use READ THE HYBRESIS PATCH AND CHARGING STATION INSTRUCTIONS FOR USE FOR ADDITIONAL IMPORTANT INFORMATION. REF: 199587 GLOSSARY OF SYMBOLS This device may contain one or
Controller - Instructions For Use READ THE HYBRESIS PATCH AND CHARGING STATION INSTRUCTIONS FOR USE FOR ADDITIONAL IMPORTANT INFORMATION. REF: 199587 GLOSSARY OF SYMBOLS This device may contain one or
DENTAL X-RAY OPERATOR'S INSTRUCTIONS. (for USA) Wall Mount Type...WK
 M 505 DENTAL X-RAY OPERATOR'S INSTRUCTIONS (for USA) Wall Mount Type...WK WARNING This X-ray equipment may be dangerous to patient and operator unless safe exposure factors, operating instructions and
M 505 DENTAL X-RAY OPERATOR'S INSTRUCTIONS (for USA) Wall Mount Type...WK WARNING This X-ray equipment may be dangerous to patient and operator unless safe exposure factors, operating instructions and
Pain Management System
 TM Pain Management System Model: ireliev Model #: ET-1313 Operating & Instruction Manual Read Before Using ireliev Pain Management System Intended Use The ireliev Pain Management System (Model # ET-1313)
TM Pain Management System Model: ireliev Model #: ET-1313 Operating & Instruction Manual Read Before Using ireliev Pain Management System Intended Use The ireliev Pain Management System (Model # ET-1313)
USER MANUAL M-200. Please take time to read these instructions before starting to use the scale. Version /06
 USER MANUAL M-200 Please take time to read these instructions before starting to use the scale Version 1.0 07/06 Contents Introduction 3 Product Specification 3 Safety Instructions 4 Explanation of Graphic
USER MANUAL M-200 Please take time to read these instructions before starting to use the scale Version 1.0 07/06 Contents Introduction 3 Product Specification 3 Safety Instructions 4 Explanation of Graphic
VN415/VO425 Specifications
 VN415/VO425 Specifications Item No. 8105231-1 01/2014 Contents VN415 Specifications... 1 1.1 Technical Standards:...1 1.2 System Requirements:...1 1.3 Included and Optional Parts...2 VO425 Specifications...
VN415/VO425 Specifications Item No. 8105231-1 01/2014 Contents VN415 Specifications... 1 1.1 Technical Standards:...1 1.2 System Requirements:...1 1.3 Included and Optional Parts...2 VO425 Specifications...
GTIN:
 GTIN: 00894912002920 TABLE OF CONTENTS Introduction...1 Features...1 Indications for Use...1 Contraindications...1 Warnings & Precautions...2 Controls...3 Operation...4 Recharging the Battery...5 Replacing
GTIN: 00894912002920 TABLE OF CONTENTS Introduction...1 Features...1 Indications for Use...1 Contraindications...1 Warnings & Precautions...2 Controls...3 Operation...4 Recharging the Battery...5 Replacing
The following languages can be found on our website: French. German. Spanish. Italian. Swedish. Portuguese. Russian. Dutch.
 Manufactured By: 5580 S. Nogales Hwy. Tucson, Az 85706 USA Telephone: 800-975-7987 Fax: 520-294-6061 www.westmedinc.com PN 74586, Rev. 10 MT Promedt Consulting GmbH Altenhofstr. 80 66386 St. Ingbert, Germany
Manufactured By: 5580 S. Nogales Hwy. Tucson, Az 85706 USA Telephone: 800-975-7987 Fax: 520-294-6061 www.westmedinc.com PN 74586, Rev. 10 MT Promedt Consulting GmbH Altenhofstr. 80 66386 St. Ingbert, Germany
BodyclockStimplusManual.qx8_Layout 1 17/08/ :53 Page 1. Body Clock Stimplus. Instructions for use
 BodyclockStimplusManual.qx8_Layout 1 17/08/2012 11:53 Page 1 Body Clock Stimplus TM Instructions for use BodyclockStimplusManual.qx8_Layout 1 17/08/2012 11:53 Page 2 Acupuncture is an ancient Chinese therapy
BodyclockStimplusManual.qx8_Layout 1 17/08/2012 11:53 Page 1 Body Clock Stimplus TM Instructions for use BodyclockStimplusManual.qx8_Layout 1 17/08/2012 11:53 Page 2 Acupuncture is an ancient Chinese therapy
Glass Electrode Meter
 Glass Electrode Meter INSTRUCTION MANUAL FOR Glass Electrode R/C Meter MODEL 2700 Serial # Date PO Box 850 Carlsborg, WA 98324 U.S.A. 360-683-8300 800-426-1306 FAX: 360-683-3525 http://www.a-msystems.com
Glass Electrode Meter INSTRUCTION MANUAL FOR Glass Electrode R/C Meter MODEL 2700 Serial # Date PO Box 850 Carlsborg, WA 98324 U.S.A. 360-683-8300 800-426-1306 FAX: 360-683-3525 http://www.a-msystems.com
Operating Manual Infrared thermometer
 Operating Manual Infrared thermometer Model:IT-121 Professional Fast Accurate 1 CONTENTS 1 I n t r o d u c t i o n................ 3 1.1Product intended use 3 2 Basic principle 3 3 Pr o d u c t f e a t
Operating Manual Infrared thermometer Model:IT-121 Professional Fast Accurate 1 CONTENTS 1 I n t r o d u c t i o n................ 3 1.1Product intended use 3 2 Basic principle 3 3 Pr o d u c t f e a t
HD STETH TM Quick Start User Guide
 Thank you and for choosing the futuristic HD Steth TM manufactured by HD Medical Inc. USA Indications for Use (IFU) HD Steth is an electronic stethoscope meant to assist a qualified clinician to capture,
Thank you and for choosing the futuristic HD Steth TM manufactured by HD Medical Inc. USA Indications for Use (IFU) HD Steth is an electronic stethoscope meant to assist a qualified clinician to capture,
M-400 M-410 M-420 M-430
 USER MANUAL M-400 M-410 M-420 M-430 Please take time to read these instructions before starting to use the scale Version 1.1 10/17 Contents Introduction 3 Product Specification 3 Safety Instructions 4
USER MANUAL M-400 M-410 M-420 M-430 Please take time to read these instructions before starting to use the scale Version 1.1 10/17 Contents Introduction 3 Product Specification 3 Safety Instructions 4
Central Blood Pressure Meter Model cbp301. Operating Manual
 Central Blood Pressure Meter Model cbp301 Operating Manual Document cbp301-009 Issue 4 September 2011 Contents Introduction... 3 Package Contents... 5 Warnings and Cautions... 6 Contraindications... 7
Central Blood Pressure Meter Model cbp301 Operating Manual Document cbp301-009 Issue 4 September 2011 Contents Introduction... 3 Package Contents... 5 Warnings and Cautions... 6 Contraindications... 7
SJM MRI Activator. Handheld Device. User's Manual. Model EX4000
 SJM MRI Activator Handheld Device User's Manual Model EX4000 Unless otherwise noted, indicates that the name is a trademark of, or licensed to, St. Jude Medical or one of its subsidiaries. ST. JUDE MEDICAL
SJM MRI Activator Handheld Device User's Manual Model EX4000 Unless otherwise noted, indicates that the name is a trademark of, or licensed to, St. Jude Medical or one of its subsidiaries. ST. JUDE MEDICAL
Draft. User s Manual. Transmitter Model EX1150
 User s Manual Merlin @home Transmitter Model EX1150 CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. 2008 St. Jude Medical Cardiac Rhythm Management Division.
User s Manual Merlin @home Transmitter Model EX1150 CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. 2008 St. Jude Medical Cardiac Rhythm Management Division.
ibed Locator Model 5212
 ibed Locator Model 5212 Connected Hospital Instructions for Use and Installation For Parts or Technical Assistance: USA: 1-800-327-0770 2011/03 5212-009-101 REV C www.stryker.com Table of Contents Symbols
ibed Locator Model 5212 Connected Hospital Instructions for Use and Installation For Parts or Technical Assistance: USA: 1-800-327-0770 2011/03 5212-009-101 REV C www.stryker.com Table of Contents Symbols
The following symbol indicates that the device is MR-unsafe:
 The following symbol indicates that the device is MR-unsafe: MR Unsafe Do not use this equipment In the MRI scan room Patient must follow the doctor s instructions and should not perform a self-assessment
The following symbol indicates that the device is MR-unsafe: MR Unsafe Do not use this equipment In the MRI scan room Patient must follow the doctor s instructions and should not perform a self-assessment
Exergen TAT 5000S RS232 TTL Supplemental Instructions for Use
 www.exergen.com/s Exergen TAT 5000S RS232 TTL Supplemental Instructions for Use For additional specifications, see GE Healthcare CARESCAPE V100 Vital Signs Monitor Operator's Manual, Section 12. Symbol
www.exergen.com/s Exergen TAT 5000S RS232 TTL Supplemental Instructions for Use For additional specifications, see GE Healthcare CARESCAPE V100 Vital Signs Monitor Operator's Manual, Section 12. Symbol
TETRIS 1000 High Impedance Active Probe. Instruction Manual
 TETRIS 1000 High Impedance Active Probe Instruction Manual Copyright 2015 PMK GmbH All rights reserved. Information in this publication supersedes that in all previously published material. Specifications
TETRIS 1000 High Impedance Active Probe Instruction Manual Copyright 2015 PMK GmbH All rights reserved. Information in this publication supersedes that in all previously published material. Specifications
36 AC/DC True RMS. Clamp Meter. P Read First: Safety Information
 36 / True RMS Clamp Meter Instruction Sheet P Read First: Safety Information To ensure safe operation and service of the meter, follow these instructions: Avoid working alone so assistance can be rendered.
36 / True RMS Clamp Meter Instruction Sheet P Read First: Safety Information To ensure safe operation and service of the meter, follow these instructions: Avoid working alone so assistance can be rendered.
Operator s Manual. PP016 Passive Probe
 Operator s Manual PP016 Passive Probe 2017 Teledyne LeCroy, Inc. All rights reserved. Unauthorized duplication of Teledyne LeCroy documentation materials is strictly prohibited. Customers are permitted
Operator s Manual PP016 Passive Probe 2017 Teledyne LeCroy, Inc. All rights reserved. Unauthorized duplication of Teledyne LeCroy documentation materials is strictly prohibited. Customers are permitted
Neo Ultrasound Module Manual
 Neo Ultrasound Module Manual Installation Instructions For complete User Operating Instructions, including Cautions, Warnings, Dangers, Indications, and Contraindications, refer to the User s Manuals.
Neo Ultrasound Module Manual Installation Instructions For complete User Operating Instructions, including Cautions, Warnings, Dangers, Indications, and Contraindications, refer to the User s Manuals.
AS SUPER 4 digital. Elektrischer Nadelstimulator / Electrical needle stimulator. Art.-Nr
 AS SUPER 4 digital Elektrischer Nadelstimulator Electrical needle stimulator Art.-Nr. 200510 Gebrauchsanweisung Instruction Manual - Art.-Nr. 101477 20 Contents Foreword...21 Purpose for use...21 Safety
AS SUPER 4 digital Elektrischer Nadelstimulator Electrical needle stimulator Art.-Nr. 200510 Gebrauchsanweisung Instruction Manual - Art.-Nr. 101477 20 Contents Foreword...21 Purpose for use...21 Safety
Transcutaneous Electrical Nerve Stimulator
 Transcutaneous Electrical Nerve Stimulator Simple, effective pain relief *For the temporary relief of pain as part of current medication programme. Distributor Medi-Direct International Ltd. Unit 24, Wilford
Transcutaneous Electrical Nerve Stimulator Simple, effective pain relief *For the temporary relief of pain as part of current medication programme. Distributor Medi-Direct International Ltd. Unit 24, Wilford
WRIST BLOOD PRESSURE MONITOR
 WRIST BLOOD PRESSURE MONITOR Instruction Manual MODEL: ABP801 www.accumed.com TABLE OF CONTENTS INTRODUCTION... 1 NOTES ON SAFETY... 1 ABOUT BLOOD PRESSURE... 3 PRECAUTIONS BEFORE US... 4 FEATURES OF THE
WRIST BLOOD PRESSURE MONITOR Instruction Manual MODEL: ABP801 www.accumed.com TABLE OF CONTENTS INTRODUCTION... 1 NOTES ON SAFETY... 1 ABOUT BLOOD PRESSURE... 3 PRECAUTIONS BEFORE US... 4 FEATURES OF THE
ENG en. Operating instructions. Iris Magneton MF Wellness therapy
 ENG en Operating instructions Iris Magneton MF Wellness therapy Edition 09 / 2012 These operating instructions constitute an accessory of the device. They must therefore be kept in a suitable place near
ENG en Operating instructions Iris Magneton MF Wellness therapy Edition 09 / 2012 These operating instructions constitute an accessory of the device. They must therefore be kept in a suitable place near
User Manual. Glass Body Fat Analyzer LS203-B. version:1.0
 version:1.0 User Manual Glass Body Fat Analyzer LS203-B Contains FCC ID: OU9AW8001-LS GUANGDONG TRANSTEK MEDICAL ELECTRONICS CO., LTD Zone A, 5/F., Investment Building, No. 12, Huizhan East Rd., Torch
version:1.0 User Manual Glass Body Fat Analyzer LS203-B Contains FCC ID: OU9AW8001-LS GUANGDONG TRANSTEK MEDICAL ELECTRONICS CO., LTD Zone A, 5/F., Investment Building, No. 12, Huizhan East Rd., Torch
TETRIS User's Guide. High Impedance Active Probe DO177-1
 TETRIS 1500 High Impedance Active Probe User's Guide DO177-1 TETRIS 1500 Copyright 2010 Ltd. All rights reserved. Information in this publication supersedes that in all previously published material. Specifications
TETRIS 1500 High Impedance Active Probe User's Guide DO177-1 TETRIS 1500 Copyright 2010 Ltd. All rights reserved. Information in this publication supersedes that in all previously published material. Specifications
INTRODUCTION. It is also intended for symptomatic relief and management of chronic, intractable pain and relief of pain associated with arthritis.
 TENS / HEAT 1 2 TABLE OF CONTENTS Introduction...4 Indications for Use...4 Safety Warning...5 Contraindications...5 Warnings...5 Precautions...6 Adverse Reactions...8 Symbol and Title...8 Environmental
TENS / HEAT 1 2 TABLE OF CONTENTS Introduction...4 Indications for Use...4 Safety Warning...5 Contraindications...5 Warnings...5 Precautions...6 Adverse Reactions...8 Symbol and Title...8 Environmental
Wireless TENS Pain Reliever. WT1 Series
 Wireless TENS Pain Reliever WT1 Series Content Introduction Parts Features General Warnings and Safety Using your Wireless TENS Pain Reliever Troubleshooting Positions for use Mode Selection Specification
Wireless TENS Pain Reliever WT1 Series Content Introduction Parts Features General Warnings and Safety Using your Wireless TENS Pain Reliever Troubleshooting Positions for use Mode Selection Specification
OPERATOR S MANUAL AN APPLIED DIGITAL COMPANY
 OPERATOR S MANUAL AN APPLIED DIGITAL COMPANY Contents: VeriChip H2 Reader Assembly Part Number 600-000515-000 (includes all of the following): USER INSTRUCTIONS Description Part Number Description The
OPERATOR S MANUAL AN APPLIED DIGITAL COMPANY Contents: VeriChip H2 Reader Assembly Part Number 600-000515-000 (includes all of the following): USER INSTRUCTIONS Description Part Number Description The
F R F R G
 w w w. e t o n c o r p. c o m F R 2 0 0 F R 2 0 0 G O P E R AT I O N M A N U A L A M / F M / S H O R T W A V E R A D I O TABLE OF CONTENTS DO YOU NEED HELP? Contact Us. Etón Corporation 1015 Corporation
w w w. e t o n c o r p. c o m F R 2 0 0 F R 2 0 0 G O P E R AT I O N M A N U A L A M / F M / S H O R T W A V E R A D I O TABLE OF CONTENTS DO YOU NEED HELP? Contact Us. Etón Corporation 1015 Corporation
Welch Allyn Home Scale (T- RPM-SCALE100)
 Welch Allyn Home Scale (T- RPM-SCALE100) Directions for use 901077 Weight scale, Software Version 1.0 2017 Welch Allyn. All rights are reserved. To support the intended use of the product described in
Welch Allyn Home Scale (T- RPM-SCALE100) Directions for use 901077 Weight scale, Software Version 1.0 2017 Welch Allyn. All rights are reserved. To support the intended use of the product described in
Ceiling Model Ceiling Model for LEDview Plus and Heliodent Plus. Installation Requirements. New as of: English
 New as of: 03.2017 Ceiling Model Ceiling Model for LEDview Plus and Heliodent Plus Installation Requirements English Installation Requirements Ceiling Version = Dentsply Sirona Installation Requirements
New as of: 03.2017 Ceiling Model Ceiling Model for LEDview Plus and Heliodent Plus Installation Requirements English Installation Requirements Ceiling Version = Dentsply Sirona Installation Requirements
User s Guide ASSISTIVE LISTENING SYSTEMS
 User s Guide ASSISTIVE LISTENING SYSTEMS 2 Digital-1 User s Guide Contents How to use Digital-1...3 Tuning...6 Frequency Chart...8 Correcting Interference...9 Recharging...10 Specifications...12 Notice...13
User s Guide ASSISTIVE LISTENING SYSTEMS 2 Digital-1 User s Guide Contents How to use Digital-1...3 Tuning...6 Frequency Chart...8 Correcting Interference...9 Recharging...10 Specifications...12 Notice...13
SPAC265-8W. AC-DC power supply module. Features. Description. Applications
 AC-DC power supply module Datasheet production data Features Open frame switch mode power supply European input voltage range Single output 5 V, 8 W peak power, 4 W continuous operating mode EMC compliance
AC-DC power supply module Datasheet production data Features Open frame switch mode power supply European input voltage range Single output 5 V, 8 W peak power, 4 W continuous operating mode EMC compliance
POCKET AIR. Portable Nebulizer. Instruction Manual MBPN002 / MB / MB05006
 R POCKET AIR Portable Nebulizer Instruction Manual MBPN002 / MB0500300/ MB05006 Table of Contents General information... 1 Intended Use... 2 Safety Precautions... 3 Explanation of Symbols... 4 Package
R POCKET AIR Portable Nebulizer Instruction Manual MBPN002 / MB0500300/ MB05006 Table of Contents General information... 1 Intended Use... 2 Safety Precautions... 3 Explanation of Symbols... 4 Package
Body Clock Stimplus Pro
 Body Clock Stimplus Pro TM Instructions for use Acupuncture is an ancient Chinese therapy in which specific points on the body, known as acupuncture points (acupoints) are stimulated by the use of needles.
Body Clock Stimplus Pro TM Instructions for use Acupuncture is an ancient Chinese therapy in which specific points on the body, known as acupuncture points (acupoints) are stimulated by the use of needles.
MODEL PCU Pressure Control Unit with Patient Isolation. Instructions for Use (IFU) Sensors.Systems.Solutions.
 World Headquarters Millar Instruments, Inc. 6001-A Gulf Freeway Houston, Texas 77023-5417 USA Phone: 832.667.7000 or 800-669-2343 (in the USA) Fax: 832.667.7001 Email: info@millarmail.com Web site: www.millarinstruments.com
World Headquarters Millar Instruments, Inc. 6001-A Gulf Freeway Houston, Texas 77023-5417 USA Phone: 832.667.7000 or 800-669-2343 (in the USA) Fax: 832.667.7001 Email: info@millarmail.com Web site: www.millarinstruments.com
Operator s Manual External Remote Controller (ERC)
 OM0000-C, 2010-07 Page 1 of 25 Table of Contents 1. SYMBOLS DEFINITION:...3 2. PRODUCT WARNINGS:...6 3. PRODUCT DESCRIPTION:...8 4. COMPONENT IDENTIFICATION:...8 5. INSTRUCTIONS FOR USE:...9 6. EMERGENCY
OM0000-C, 2010-07 Page 1 of 25 Table of Contents 1. SYMBOLS DEFINITION:...3 2. PRODUCT WARNINGS:...6 3. PRODUCT DESCRIPTION:...8 4. COMPONENT IDENTIFICATION:...8 5. INSTRUCTIONS FOR USE:...9 6. EMERGENCY
For detailed specifications and ordering info go to
 For detailed specifications and ordering info go to www.testequipmentdepot.com ECB50A, ECB50A-E, ECB50A-FGIS Circuit Breaker Finder and AC Cable Tracer User Manual For detailed specifications and ordering
For detailed specifications and ordering info go to www.testequipmentdepot.com ECB50A, ECB50A-E, ECB50A-FGIS Circuit Breaker Finder and AC Cable Tracer User Manual For detailed specifications and ordering
By Paul Aylett at 12:08 pm, Apr 13, 2016
 TD2 Series Content Introduction Parts Features General Warnings and Safety Using your TENS Pain Reliever Troubleshooting Positions for use Specification Compatibility EMC Maintenance and Cautions Explanation
TD2 Series Content Introduction Parts Features General Warnings and Safety Using your TENS Pain Reliever Troubleshooting Positions for use Specification Compatibility EMC Maintenance and Cautions Explanation
L NKTEMP. Non-contact. Infrared Thermometer. User Manual LMP001
 L NKTEMP Non-contact Infrared Thermometer User Manual LMP001 TABLE OF CONTENTS Product Description Intended Use Product Features Safety Precautions Using the Thermometer Taking a Measurement Temperature
L NKTEMP Non-contact Infrared Thermometer User Manual LMP001 TABLE OF CONTENTS Product Description Intended Use Product Features Safety Precautions Using the Thermometer Taking a Measurement Temperature
This manual is valid for the TM. In TENSity 5000 TENS Stimulator. This user manual is published by Current Solutions, LLC
 INSTRUCTION MANUAL This manual is valid for the TM In TENSity 5000 TENS Stimulator This user manual is published by Current Solutions, LLC Current Solutions, LLC does not guarantee its contents and reserves
INSTRUCTION MANUAL This manual is valid for the TM In TENSity 5000 TENS Stimulator This user manual is published by Current Solutions, LLC Current Solutions, LLC does not guarantee its contents and reserves
PHV RO High Voltage Passive Probe. Instruction Manual
 PHV 1000-3-RO High Voltage Passive Probe Instruction Manual Copyright 2012 PMK GmbH All rights reserved. Information in this publication supersedes that in all previously published material. Specifications
PHV 1000-3-RO High Voltage Passive Probe Instruction Manual Copyright 2012 PMK GmbH All rights reserved. Information in this publication supersedes that in all previously published material. Specifications
Acu-Park TM. user s guide Directed Electronics, Inc. Vista, CA N9100T 09-04
 Acu-Park TM user s guide 2004 Directed Electronics, Inc. Vista, CA N9100T 09-04 limited one year warranty Directed Electronics, Inc. (hereinafter "Directed") promises to the original purchaser that this
Acu-Park TM user s guide 2004 Directed Electronics, Inc. Vista, CA N9100T 09-04 limited one year warranty Directed Electronics, Inc. (hereinafter "Directed") promises to the original purchaser that this
User s Manual. MiniTec TM Series. Model MN26 (Model MN26T includes temperature probe) Mini Autoranging MultiMeter
 User s Manual MiniTec TM Series Model MN26 (Model MN26T includes temperature probe) Mini Autoranging MultiMeter Introduction Congratulations on your purchase of Extech s MN26 Autoranging Multimeter. This
User s Manual MiniTec TM Series Model MN26 (Model MN26T includes temperature probe) Mini Autoranging MultiMeter Introduction Congratulations on your purchase of Extech s MN26 Autoranging Multimeter. This
AT Advanced Wire Tracer. Users Manual
 AT-1000 Advanced Wire Tracer Users Manual AT-1000 Advanced Wire Tracer English Users Manual AT1000_Rev001 2008 Amprobe Test Tools. All rights reserved. Limited Warranty and Limitation of Liability Your
AT-1000 Advanced Wire Tracer Users Manual AT-1000 Advanced Wire Tracer English Users Manual AT1000_Rev001 2008 Amprobe Test Tools. All rights reserved. Limited Warranty and Limitation of Liability Your
2001A. 200KHz Function Generator Instruction Manual. 99 Washington Street Melrose, MA Phone Toll Free
 2001A 200KHz Function Generator Instruction Manual 99 Washington Street Melrose, MA 02176 Phone 781-665-1400 Toll Free 1-800-517-8431 Visit us at www.testequipmentdepot.com WARRANTY Global Specialties
2001A 200KHz Function Generator Instruction Manual 99 Washington Street Melrose, MA 02176 Phone 781-665-1400 Toll Free 1-800-517-8431 Visit us at www.testequipmentdepot.com WARRANTY Global Specialties
Model 5100F. Advanced Test Equipment Rentals ATEC (2832) OWNER S MANUAL RF POWER AMPLIFIER
 Established 1981 Advanced Test Equipment Rentals www.atecorp.com 800-404-ATEC (2832) OWNER S MANUAL Model 5100F RF POWER AMPLIFIER 0.8 2.5 GHz, 25 Watts Ophir RF 5300 Beethoven Street Los Angeles, CA 90066
Established 1981 Advanced Test Equipment Rentals www.atecorp.com 800-404-ATEC (2832) OWNER S MANUAL Model 5100F RF POWER AMPLIFIER 0.8 2.5 GHz, 25 Watts Ophir RF 5300 Beethoven Street Los Angeles, CA 90066
H2 Check Operating Manual
 H2 Check Operating Manual Federal (USA) law restricts this device to sale by or on the order of a physician or licensed practitioner. Micro Direct, Inc. 803 Webster Street Lewiston, ME 04240 1-800-588-3381
H2 Check Operating Manual Federal (USA) law restricts this device to sale by or on the order of a physician or licensed practitioner. Micro Direct, Inc. 803 Webster Street Lewiston, ME 04240 1-800-588-3381
PKT 512A-RO High Impedance Passive Cable Divider
 PKT 512A-RO High Impedance Passive Cable Divider Instruction Manual Copyright 2011 PMK GmbH All rights reserved. Information in this publication supersedes that in all previously published material. Specifications
PKT 512A-RO High Impedance Passive Cable Divider Instruction Manual Copyright 2011 PMK GmbH All rights reserved. Information in this publication supersedes that in all previously published material. Specifications
Osmolarity System USER MANUAL FOR QUANTITATIVE MEASUREMENT OF OSMOLARITY OF OCULAR TISSUES. osdcare.com
 Osmolarity System FOR QUANTITATIVE MEASUREMENT OF OSMOLARITY OF OCULAR TISSUES USER MANUAL osdcare.com I-PEN User Guide I-PEN User Guide TABLE OF CONTENTS I-PEN I-PEN is a trademark of I-MED Pharma Inc.
Osmolarity System FOR QUANTITATIVE MEASUREMENT OF OSMOLARITY OF OCULAR TISSUES USER MANUAL osdcare.com I-PEN User Guide I-PEN User Guide TABLE OF CONTENTS I-PEN I-PEN is a trademark of I-MED Pharma Inc.
PHV 1000-RO High Voltage Passive Probe. Instruction Manual
 PHV 1000-RO High Voltage Passive Probe Instruction Manual Copyright 2014 PMK GmbH All rights reserved. Information in this publication supersedes that in all previously published material. Specifications
PHV 1000-RO High Voltage Passive Probe Instruction Manual Copyright 2014 PMK GmbH All rights reserved. Information in this publication supersedes that in all previously published material. Specifications
User s Guide. Model MA A AC Mini Clamp-on Meter
 User s Guide Model MA150 200A AC Mini Clamp-on Meter Introduction Congratulations on your purchase of Extech s MA150 AC Mini Clamp Meter. This meter is shipped fully tested and calibrated and, with proper
User s Guide Model MA150 200A AC Mini Clamp-on Meter Introduction Congratulations on your purchase of Extech s MA150 AC Mini Clamp Meter. This meter is shipped fully tested and calibrated and, with proper
MINISCAV Vacuum Pump Manual
 MINISCAV Vacuum Pump Manual North American Model Caution: Federal law restricts this device to sale by or on the order of a physician or dentist. MR Unsafe - Do not place or use Miniscav in or near an
MINISCAV Vacuum Pump Manual North American Model Caution: Federal law restricts this device to sale by or on the order of a physician or dentist. MR Unsafe - Do not place or use Miniscav in or near an
HHMA2 DC / TRUE RMS AC NON-CONTACT MILLIAMMETER
 HHMA2 DC / TRUE RMS AC NON-CONTACT MILLIAMMETER Instruction Manual Manual UN-01-249 Item 359934 April, 1999 Rev. -- OMEGA Engineering Inc. All rights reserved. This symbol appears on the instrument and
HHMA2 DC / TRUE RMS AC NON-CONTACT MILLIAMMETER Instruction Manual Manual UN-01-249 Item 359934 April, 1999 Rev. -- OMEGA Engineering Inc. All rights reserved. This symbol appears on the instrument and
Compressor Nebulizer. Model No: 9R-021 series. Please read the instruction manual before use.
 Compressor Nebulizer Model No: 9R-021 series Please read the instruction manual before use. IMPORTANT SAFEGUARDS...... 1 1. INTRODUCTION... 6 2. PRODUCT DESCRIPTION... 7 3. OPERATION... 8 4. CLEANING...
Compressor Nebulizer Model No: 9R-021 series Please read the instruction manual before use. IMPORTANT SAFEGUARDS...... 1 1. INTRODUCTION... 6 2. PRODUCT DESCRIPTION... 7 3. OPERATION... 8 4. CLEANING...
This product may malfunction due to electromagnetic waves caused by portable
 1 IMPORTANT NOTICE This product may malfunction due to electromagnetic waves caused by portable personal telephones, transceivers, radio-controlled toys, etc. Be sure to avoid having objects such as, which
1 IMPORTANT NOTICE This product may malfunction due to electromagnetic waves caused by portable personal telephones, transceivers, radio-controlled toys, etc. Be sure to avoid having objects such as, which
Multi-Control Panel for built-in system. Multi Pad for NLX / imd OPERATION MANUAL OM-E0538E 001
 Multi-Control Panel for built-in system Multi Pad for NLX / imd OPERATION MANUAL OM-E0538E 001 Thank you for purchasing Multi Pad. Read this Operation Manual carefully before use for operation instructions
Multi-Control Panel for built-in system Multi Pad for NLX / imd OPERATION MANUAL OM-E0538E 001 Thank you for purchasing Multi Pad. Read this Operation Manual carefully before use for operation instructions
Schooners II. Weatherproof Wireless 900MHz Speaker System. User Guide. Model no.: GDI-AQSHR200 / AQSHR21
 Schooners II Weatherproof Wireless 900MHz Speaker System User Guide Model no.: GDI-AQSHR200 / AQSHR21 IMPORTANT: Please read your User s Guide before using your system INTRODUCTION Your SCHOONERS II speaker
Schooners II Weatherproof Wireless 900MHz Speaker System User Guide Model no.: GDI-AQSHR200 / AQSHR21 IMPORTANT: Please read your User s Guide before using your system INTRODUCTION Your SCHOONERS II speaker
Transmitter Model EX1100, EX1100W. User's Manual
 Merlin@home Transmitter Model EX1100, EX1100W User's Manual Unless otherwise noted, indicates that the name is a trademark of, or licensed to, St. Jude Medical or one of its subsidiaries. ST. JUDE MEDICAL
Merlin@home Transmitter Model EX1100, EX1100W User's Manual Unless otherwise noted, indicates that the name is a trademark of, or licensed to, St. Jude Medical or one of its subsidiaries. ST. JUDE MEDICAL
Pocket Weatheradio with Tone and Vibrating Alert
 Pocket Weatheradio with Tone and Vibrating Alert OWNER S MANUAL Please read before using this equipment. Your RadioShack Pocket Weatheradio is designed to receive National Weather Service (NWS) broadcasts,
Pocket Weatheradio with Tone and Vibrating Alert OWNER S MANUAL Please read before using this equipment. Your RadioShack Pocket Weatheradio is designed to receive National Weather Service (NWS) broadcasts,
200Amp AC Clamp Meter + NCV Model MA250
 User's Guide 200Amp AC Clamp Meter + NCV Model MA250 Introduction Congratulations on your purchase of this Extech MA250 Clamp Meter. This meter measures AC Current, AC/DC Voltage, Resistance, Capacitance,
User's Guide 200Amp AC Clamp Meter + NCV Model MA250 Introduction Congratulations on your purchase of this Extech MA250 Clamp Meter. This meter measures AC Current, AC/DC Voltage, Resistance, Capacitance,
TransAeris. System User Manual
 TransAeris System User Manual 2 This page left intentionally blank The following list includes trademarks or registered trademarks of Synapse Biomedical in the United States and possibly in other countries.
TransAeris System User Manual 2 This page left intentionally blank The following list includes trademarks or registered trademarks of Synapse Biomedical in the United States and possibly in other countries.
Assistive Listening Systems. RX-6 User s Guide
 Assistive Listening Systems RX-6 User s Guide Page ii RX-6 User s Guide Copyright Information Contents Introduction 1 Controls 2 Installing Batteries 3 Operation 3 Tuning the RX-6 4 Changing Preset Channels
Assistive Listening Systems RX-6 User s Guide Page ii RX-6 User s Guide Copyright Information Contents Introduction 1 Controls 2 Installing Batteries 3 Operation 3 Tuning the RX-6 4 Changing Preset Channels
INSTRUCTION MANUAL FOR MICROELECTRODE AC AMPLIFIER MODEL 1800
 INSTRUCTION MANUAL FOR MICROELECTRODE AC AMPLIFIER MODEL 1800 Serial # Date, Inc. PO Box 850 Carlsborg, WA 98324 U.S.A. 360-683-8300 800-426-1306 FAX: 360-683-3525 http://www.a-msystems.com Version 9.0
INSTRUCTION MANUAL FOR MICROELECTRODE AC AMPLIFIER MODEL 1800 Serial # Date, Inc. PO Box 850 Carlsborg, WA 98324 U.S.A. 360-683-8300 800-426-1306 FAX: 360-683-3525 http://www.a-msystems.com Version 9.0
ELECTROSURGICAL UNIT ANALYZER
 ELECTROSURGICAL UNIT ANALYZER ESU-2000A USER MANUAL BC BIOMEDICAL ESU-2000A TABLE OF CONTENTS WARNINGS, CAUTIONS, NOTICES... ii DESCRIPTION... 1 OVERVIEW... 2 OPERATING INSTRUCTIONS... 3 MANUAL REVISIONS...
ELECTROSURGICAL UNIT ANALYZER ESU-2000A USER MANUAL BC BIOMEDICAL ESU-2000A TABLE OF CONTENTS WARNINGS, CAUTIONS, NOTICES... ii DESCRIPTION... 1 OVERVIEW... 2 OPERATING INSTRUCTIONS... 3 MANUAL REVISIONS...
OPERATOR'S INSTRUCTIONS DENTAL X-RAY MODEL 096
 DENTAL X-RAY MODEL 096 OPERATOR'S INSTRUCTIONS 0197 WARNING: This X-ray equipment may be dangerous to patients and operators unless safe exposure factors and operating instructions are observed. R Table
DENTAL X-RAY MODEL 096 OPERATOR'S INSTRUCTIONS 0197 WARNING: This X-ray equipment may be dangerous to patients and operators unless safe exposure factors and operating instructions are observed. R Table
34134A AC/DC DMM Current Probe. User s Guide. Publication number April 2009
 User s Guide Publication number 34134-90001 April 2009 For Safety information, Warranties, Regulatory information, and publishing information, see the pages at the back of this book. Copyright Agilent
User s Guide Publication number 34134-90001 April 2009 For Safety information, Warranties, Regulatory information, and publishing information, see the pages at the back of this book. Copyright Agilent
