Overview and Version 3.1.0
|
|
|
- Angelica Harris
- 5 years ago
- Views:
Transcription
1 Overview and Version 3.1.0
2 The sponsor and the investigator shall keep a clinical trial master file. The clinical trial master file shall at all times contain the essential documents relating to that clinical trial which allow verification of the conduct of a clinical trial and the quality of the data generated [ ]. It shall be readily available, and directly accessible upon request, to the Member States. [EU Regulation 536/2014]
3 Essential documents are those documents that individually and collectively permit evaluation of the conduct of a trial and the quality of the data produced. These documents serve to demonstrate the compliance of the investigator, sponsor, and monitor with the standards of GCP and with all applicable regulatory requirements. [ICH GCP, Section 8.1]
4 ICH GCP Section The minimum list of essential documents that has been developed... ICH GCP does NOT provide a comprehensive contents list for the TMF Examples of missing documentation: Electronic systems Data management and statistical methodology Safety monitoring
5 DIA Communities Document & Records Management Community EDM Reference Model Gap in Electronic Document Management (EDM) Reference Model identified for nonsubmission TMF documents EDM scope is regulatory submissions: Significant input to the EDM Reference Model is TMF Documents Hence the creation of the TMF Reference Model 5
6 Minimum list of essential documents, as defined by ICH GCP, Chapter 8 Supporting files e.g. computer SDLC files; GMP manufacturing files; vendor selection files Usually considered outside the scope of the TMF Other trial-related records that permit evaluation of the conduct of the trial and quality of data produced The Trial Master File Other business records
7 Standardizes company content and structure and limits company customization We all follow the same regulatory requirements Inspectors are the same across companies Company-specific requirements are often driven by tradition, legacy or personal opinion Simplifies engagement of CROs and other third parties Simplifies consolidation of disparate documents into a single TMF structure (in real time, at defined trial events and/or at study end) 7
8 Managed by a Steering Committee of 14 members Change Control Board Extended Group of over 700 members Mar 2009: 1 st Meeting June 2010: Version zones w/ associated artifacts Nov 2011: Version 1.2- Investigator Site File and 1 st Intro slide set ~Dec 2012: Kickoff of many Work Groups that support the TMF manage-ment process June 2015: Release of version 3 October 2018: Release of version Feb 2011: Version 1.1- Regulator feedback June 2012: Version 2.0- Device, Process-based metadata, and IIS Feb 2014: Establishment of the TMF RM Steering Committee June 2018: Release of The Exchange Mechanism 8
9 Standard Contents Industry opinion on what is kept in a TMF Standard Naming Based on ICH E6 Sect. 8 & industry-accepted terminology Standard Structure To support paper and electronic systems Standard Metadata For etmfs, minimum metadata at system and artifact level 21 9
10 Standard Contents Industry opinion on what is kept in a TMF Expands minimum list of documents found in ICH GCP Consistent interpretation, based on peer opinion and regulator feedback Avoids scope creep for TMF 10
11 Standard Naming Based on ICH E6 Sect. 8 & industry-accepted terminology Avoids one artifact being referred to using different terms within an organisation and between organisations Avoids companyspecific terms 11
12 Standard Structure To support paper and electronic systems Facilitates consistent filing and rapid retrieval Helpful when responsibility for maintaining different sections of the TMF is distributed across several parties e.g. sponsor, CRO, consultants 12
13 Standard Metadata For etmfs, minimum metadata at system and artifact level Encourages adoption of good practices to facilitate document retrieval Encourages consistency across the industry for exchange of content 13
14 Data held in a simple Excel spreadsheet Easy for non-technical people to use! Hierarchical structure 11 Zones 48 Sections 249 Artifacts 14
15 11 Zones Trial Management Central Trial Documents Regulatory IRB or IEC and other Approvals Site Management IP and Trial Supplies Safety Reporting Central and Local Testing Third Parties Data Management Statistics 27 15
16 The contents of each zone are grouped into sections Each section includes content that is relevant to a specified activity Sections are helpful for classification and searching 16
17 Could include data files, documents, media, digitised content Could be 1 document or multiple documents Includes associated records e.g. approvals, translations, checklists, QC records, amendments 17
18 A description to explain the content of an artifact and/or the use and purpose of the artifact Assists with ensuring a common interpretation of the model Aligned with ICH definitions 18
19 Reference to the ICH GCP Guidelines Notice that other sections beyond E6 Section 8 are quoted Includes indirect as well as direct references 19
20 When an artifact name does not explicitly refer to a single kind of record (e.g. Meeting Material), sub-artifacts provide a means to list all company-specific records that are expected for a given artifact. Only examples are provided in the model but expected to be overridden as part of adopting the Reference Model for a company. Current subgroup activity to refine 20
21 To create a paper TMF, split the Model out to 3 spreadsheets, filtering for trial, country and site on each 21
22 Maintenance release e.g. v3.0.1 e.g. minor typographic changes, clarification, sub-artifacts Minor release e.g. v3.1.0 Substantial change in content but no compatibility issues e.g. additional optional column (milestones) Major release e.g. v4.0.0 Change likely to have compatibility issues with prior version e.g. addition/removal of artifacts 39
23 Documentation Delivered TMF Reference Model Version TMF Reference Model Version Release Notes that provides details on changes Released on 10-Sep-2018 for preview Effective as of 10-Oct Change Requests By the Numbers Total of 64 Change Requests Submitted since October Approved and included in release Rejected 21 Deferred Deferred to sub-teams, Steering Committee or next release
24 Added deliverables already approved Suggested dating conventions for each artifact (Feb 2017) Recommended milestones/events (Jan 2018) Also scheduled for assessment during 2019 to take account of industry feedback
25 Four minor changes to artifact name Regulatory Approval Notification. Regulatory Approval Decision Import or Export License. Import or Export Documentation Notification to Regulatory Authority of Safety or Trial Information. Notification of Safety or Trial Information Data QC or QA Plan and Results. Data Review Documentation
26 Eight minor changes to artifact definition/purpose Filenote Investigator s Brochure Regulatory Submission Notification of Safety or Study Information IP Transfer Documentation Record of Retained Samples Analysis QC Documentation Final Analysis Datasets
27 Sub-artifacts added for three artifacts Dictionary Coding Data QC or QA Plan and Results Clinical Study Report Further sub-artifacts currently under development by subartifact team. for release in 2019
28 Two artifacts with revised ICH codes To correct a typographical error Protocol Protocol Amendment
29 Three artifacts with additional filing level Added study-level: Regulatory Submission Regulatory Approval Notification Added site level: IP Unblinding Plan
30 Two artifacts with additional alternate names To correct a typographical error Regulatory Authority Decision Import or Export Documentation
31 If you have any feedback on the TMF Reference Model, including comments on existing artifacts, milestones, suggestions for additional artifacts or general comments about the TMF Reference Model, please use the link below to submit your feedback:
32 Use online form for: Making a suggestion for a general enhancement to the Reference Model Suggesting a change to any metadata for an existing artifact Suggesting a new artifact Select the appropriate option and only make ONE suggestion per form submitted please. Do not send general queries using this form.
33 Have a passion for the TMF? Are you an expert in a particular area? We are always looking for new members to join the zone teams! Follow the instructions on the Join page or contact any member of the CCB team
34 What is the Impact? Release notes give all details to assess impact Minor release so minimal impact on overall structure Artifact names may change BUT the artifact numbers do not change Includes process aspects such as milestones and dating conventions very customised by Sponsors / CROs
35 Minor/Maintenance release anticipated in 1Q 2019 Major release anticipated later in 2019 to incorporate deliverables from the following sub-teams: Sub-artifact Observational and Device
36 40
37 Group Metadata Sub-artifacts Aim To standardise the metadata collected integrated into the Exchange Mechanism To standardise the subartifacts in the TMF RM Observational Studies Device Studies JGCP Artifacts specific to observational studies Device specific artifacts Mapping to Japanese GCP documents 42
38 QUESTIONS? Join the TMF Reference Model Yahoo! Discussion Group Knowledge sharing Networking Too Much Fun! Join the TMF Reference Model Project Team
Meeting details reminder TMF RM Community update Subgroup Activity. Version Upcoming Industry meetings. Welcome
 10 September 2018 Welcome Meeting details reminder TMF RM Community update Subgroup Activity Sub-artifact Exchange mechanism Framework for destruction Version 3.1.0 Upcoming Industry meetings 10 new project
10 September 2018 Welcome Meeting details reminder TMF RM Community update Subgroup Activity Sub-artifact Exchange mechanism Framework for destruction Version 3.1.0 Upcoming Industry meetings 10 new project
Quality and GLP for Histology and Pathology of Drug Safety Studies
 Quality and GLP for Histology and Pathology of Drug Safety Studies Roger Alison BVSc MRCVS DiplECVP Consultant Toxicological Pathologist What is Quality Histology? It depends upon the purpose - Answer
Quality and GLP for Histology and Pathology of Drug Safety Studies Roger Alison BVSc MRCVS DiplECVP Consultant Toxicological Pathologist What is Quality Histology? It depends upon the purpose - Answer
European Update. 3 rd December 2015
 European Update Place your chosen image here. The four corners must just cover the arrow tips. For covers, the three pictures should be the same size and in a straight line. 3 rd December 2015 1. General
European Update Place your chosen image here. The four corners must just cover the arrow tips. For covers, the three pictures should be the same size and in a straight line. 3 rd December 2015 1. General
Software as a Medical Device (SaMD)
 Software as a Medical Device () Working Group Status Application of Clinical Evaluation Working Group Chair: Bakul Patel Center for Devices and Radiological Health US Food and Drug Administration NWIE
Software as a Medical Device () Working Group Status Application of Clinical Evaluation Working Group Chair: Bakul Patel Center for Devices and Radiological Health US Food and Drug Administration NWIE
Meeting of International Authorities under the Patent Cooperation Treaty (PCT)
 E ORIGINAL: ENGLISH ONLY DATE: JANUARY 17, 2013 Meeting of International Authorities under the Patent Cooperation Treaty (PCT) Twentieth Session Munich, February 6 to 8, 2013 QUALITY Document prepared
E ORIGINAL: ENGLISH ONLY DATE: JANUARY 17, 2013 Meeting of International Authorities under the Patent Cooperation Treaty (PCT) Twentieth Session Munich, February 6 to 8, 2013 QUALITY Document prepared
European Update. 5 th May 2016
 European Update Place your chosen image here. The four corners must just cover the arrow tips. For covers, the three pictures should be the same size and in a straight line. 5 th May 2016 1. General Update
European Update Place your chosen image here. The four corners must just cover the arrow tips. For covers, the three pictures should be the same size and in a straight line. 5 th May 2016 1. General Update
the SPD company Dr Clive Simon, Principal, The SPD Company.
 the SPD company With decades of local and international experience at the coalface, The SPD Company s specialists have built a solid repertoire of in-depth technical knowledge within the highly-regulated
the SPD company With decades of local and international experience at the coalface, The SPD Company s specialists have built a solid repertoire of in-depth technical knowledge within the highly-regulated
Revision of BS10175:2001. The proposed changes. SCI Consultation Event July 14, Richard Owen
 Revision of BS10175:2001 The proposed changes SCI Consultation Event July 14, 2010 Richard Owen Topics Covered Objectives of the revision Revision programme Methodology adopted Extent of changes - general
Revision of BS10175:2001 The proposed changes SCI Consultation Event July 14, 2010 Richard Owen Topics Covered Objectives of the revision Revision programme Methodology adopted Extent of changes - general
ENCePP Work Plan
 EMA/231507/2012 ENCePP Secretariat European Network of Centres for Pharmacoepidemiology and Pharmacovigilance European Network of Centres for Pharmacoepidemiology and Pharmacovigilance Adopted by the ENCePP
EMA/231507/2012 ENCePP Secretariat European Network of Centres for Pharmacoepidemiology and Pharmacovigilance European Network of Centres for Pharmacoepidemiology and Pharmacovigilance Adopted by the ENCePP
Safety related product corrective action
 Safety related product corrective action Brian Such Standards Solutions Project Manager British Standards Institution Copyright 2017 BSI. All rights reserved 1 03/07/2017 Safety related product corrective
Safety related product corrective action Brian Such Standards Solutions Project Manager British Standards Institution Copyright 2017 BSI. All rights reserved 1 03/07/2017 Safety related product corrective
EU, USA and Japan (II) Reports from Regulators on Exchange Assignments
 EU, USA and Japan (II) Reports from Regulators on Exchange Assignments Yoshikazu Hayashi MHLW/PMDA Liaison at EMA PMDA 24th Annual EuroMeeting 26-28 March 2012 Copenhagen, Denmark Disclaimer The views
EU, USA and Japan (II) Reports from Regulators on Exchange Assignments Yoshikazu Hayashi MHLW/PMDA Liaison at EMA PMDA 24th Annual EuroMeeting 26-28 March 2012 Copenhagen, Denmark Disclaimer The views
SAUDI ARABIAN STANDARDS ORGANIZATION (SASO) TECHNICAL DIRECTIVE PART ONE: STANDARDIZATION AND RELATED ACTIVITIES GENERAL VOCABULARY
 SAUDI ARABIAN STANDARDS ORGANIZATION (SASO) TECHNICAL DIRECTIVE PART ONE: STANDARDIZATION AND RELATED ACTIVITIES GENERAL VOCABULARY D8-19 7-2005 FOREWORD This Part of SASO s Technical Directives is Adopted
SAUDI ARABIAN STANDARDS ORGANIZATION (SASO) TECHNICAL DIRECTIVE PART ONE: STANDARDIZATION AND RELATED ACTIVITIES GENERAL VOCABULARY D8-19 7-2005 FOREWORD This Part of SASO s Technical Directives is Adopted
ENGINEERING DRAWINGS MANAGEMENT POLICY (IFC/AS BUILTS)
 Approval Amendment Record Approval Date Version Description 15/10/2015 1 This policy takes precedence over L1-NAM-PRO-003 Infrastructure As Built Drawing Management due to business restructures. New Content
Approval Amendment Record Approval Date Version Description 15/10/2015 1 This policy takes precedence over L1-NAM-PRO-003 Infrastructure As Built Drawing Management due to business restructures. New Content
SHTG primary submission process
 Meeting date: 24 April 2014 Agenda item: 8 Paper number: SHTG 14-16 Title: Purpose: SHTG primary submission process FOR INFORMATION Background The purpose of this paper is to update SHTG members on developments
Meeting date: 24 April 2014 Agenda item: 8 Paper number: SHTG 14-16 Title: Purpose: SHTG primary submission process FOR INFORMATION Background The purpose of this paper is to update SHTG members on developments
EDQM COUNCIL OF EUROPE CONFERENCE CERTIFICATION PROCEDURE : 20 YEARS OF EXPERIENCE March EDQM, Strasbourg, France ABSTRACTS
 EDQM COUNCIL OF EUROPE CONFERENCE CERTIFICATION PROCEDURE 1992-2012: 20 YEARS OF EXPERIENCE 22-23 March 2012 EDQM, Strasbourg, France ABSTRACTS PLENARY SESSION, 22 March 2012 ABSTRACT 1.3 The Evolution
EDQM COUNCIL OF EUROPE CONFERENCE CERTIFICATION PROCEDURE 1992-2012: 20 YEARS OF EXPERIENCE 22-23 March 2012 EDQM, Strasbourg, France ABSTRACTS PLENARY SESSION, 22 March 2012 ABSTRACT 1.3 The Evolution
TERMS OF REFERENCE FOR CONSULTANTS
 Strengthening Systems for Promoting Science, Technology, and Innovation (KSTA MON 51123) TERMS OF REFERENCE FOR CONSULTANTS 1. The Asian Development Bank (ADB) will engage 77 person-months of consulting
Strengthening Systems for Promoting Science, Technology, and Innovation (KSTA MON 51123) TERMS OF REFERENCE FOR CONSULTANTS 1. The Asian Development Bank (ADB) will engage 77 person-months of consulting
SECTION SUBMITTAL PROCEDURES
 SECTION 01330 - SUBMITTAL PROCEDURES PART 1 - GENERAL 1.1 RELATED DOCUMENTS A. Drawings and general provisions of the Contract, including General and Supplementary Conditions and other Division 1 Specification
SECTION 01330 - SUBMITTAL PROCEDURES PART 1 - GENERAL 1.1 RELATED DOCUMENTS A. Drawings and general provisions of the Contract, including General and Supplementary Conditions and other Division 1 Specification
Health Based Exposure Limits (HBEL) and Q&As
 Health Based Exposure Limits (HBEL) and Q&As The EMA guideline (EMA/CHMP/ CVMP/ SWP/169430/2012) & EMA/CHMP/CVMP/SWP/463311/2016 Graeme McKilligan, UK, MHRA. Content Intent of HBEL Post Implementation
Health Based Exposure Limits (HBEL) and Q&As The EMA guideline (EMA/CHMP/ CVMP/ SWP/169430/2012) & EMA/CHMP/CVMP/SWP/463311/2016 Graeme McKilligan, UK, MHRA. Content Intent of HBEL Post Implementation
June Phase 3 Executive Summary Pre-Project Design Review of Candu Energy Inc. Enhanced CANDU 6 Design
 June 2013 Phase 3 Executive Summary Pre-Project Design Review of Candu Energy Inc. Enhanced CANDU 6 Design Executive Summary A vendor pre-project design review of a new nuclear power plant provides an
June 2013 Phase 3 Executive Summary Pre-Project Design Review of Candu Energy Inc. Enhanced CANDU 6 Design Executive Summary A vendor pre-project design review of a new nuclear power plant provides an
The European Securitisation Regulation: The Countdown Continues... Draft Regulatory Technical Standards on Content and Format of the STS Notification
 WHITE PAPER March 2018 The European Securitisation Regulation: The Countdown Continues... Draft Regulatory Technical Standards on Content and Format of the STS Notification Regulation (EU) 2017/2402, which
WHITE PAPER March 2018 The European Securitisation Regulation: The Countdown Continues... Draft Regulatory Technical Standards on Content and Format of the STS Notification Regulation (EU) 2017/2402, which
Outline of Presentation
 WHAT IS VALUE IN HEALTH DOING FOR ITS AUTHORS? Michael Drummond C. Daniel Mullins Co-Editors-in-Chief Value in Health Outline of Presentation Scope and Overview of Value in Health What Value in Health
WHAT IS VALUE IN HEALTH DOING FOR ITS AUTHORS? Michael Drummond C. Daniel Mullins Co-Editors-in-Chief Value in Health Outline of Presentation Scope and Overview of Value in Health What Value in Health
Appendix-1. Project Design Matrix (PDM)
 Appendix-1 Project Design Matrix (PDM) Appendix-I Project Design Matrix (PDM) Version 1 PDM: Electric Power Technical Standards Promotion Project in Vietnam Duration: 3 Years (March in 2010 to January
Appendix-1 Project Design Matrix (PDM) Appendix-I Project Design Matrix (PDM) Version 1 PDM: Electric Power Technical Standards Promotion Project in Vietnam Duration: 3 Years (March in 2010 to January
COURSE SCHEDULE
 2018-2019 COURSE SCHEDULE Fall 1 Seven-Week: Aug. 27 Oct. 12, 2018 Business Formation & Structure -11:40am Legal & Regulatory Process ` Patent Business Formation & Structure -11:40am Research in, Business
2018-2019 COURSE SCHEDULE Fall 1 Seven-Week: Aug. 27 Oct. 12, 2018 Business Formation & Structure -11:40am Legal & Regulatory Process ` Patent Business Formation & Structure -11:40am Research in, Business
ENCePP Work Plan
 EMA/150117/2014 ENCePP Secretariat European Network of Centres for Pharmacoepidemiology and Pharmacovigilance European Network of Centres for Pharmacoepidemiology and Pharmacovigilance Adopted by the ENCePP
EMA/150117/2014 ENCePP Secretariat European Network of Centres for Pharmacoepidemiology and Pharmacovigilance European Network of Centres for Pharmacoepidemiology and Pharmacovigilance Adopted by the ENCePP
CMC Topics and PMDA s activities Yoshihiro Matsuda, Ph.D.
 CMC Topics and PMDA s activities Yoshihiro Matsuda, Ph.D. Senior Scientist (for Quality) Pharmaceuticals and Medical Devices Agency (PMDA) MFDS Nov 11, 2016 1 Agenda Introduction of PMDA ICH Q12 QbD assessment
CMC Topics and PMDA s activities Yoshihiro Matsuda, Ph.D. Senior Scientist (for Quality) Pharmaceuticals and Medical Devices Agency (PMDA) MFDS Nov 11, 2016 1 Agenda Introduction of PMDA ICH Q12 QbD assessment
Phase 2 Executive Summary: Pre-Project Review of AECL s Advanced CANDU Reactor ACR
 August 31, 2009 Phase 2 Executive Summary: Pre-Project Review of AECL s Advanced CANDU Reactor ACR-1000-1 Executive Summary A vendor pre-project design review of a new nuclear power plant provides an opportunity
August 31, 2009 Phase 2 Executive Summary: Pre-Project Review of AECL s Advanced CANDU Reactor ACR-1000-1 Executive Summary A vendor pre-project design review of a new nuclear power plant provides an opportunity
CARRA PUBLICATION AND PRESENTATION GUIDELINES Version April 20, 2017
 CARRA PUBLICATION AND PRESENTATION GUIDELINES Version April 20, 2017 1. Introduction The goals of the CARRA Publication and Presentation Guidelines are to: a) Promote timely and high-quality presentation
CARRA PUBLICATION AND PRESENTATION GUIDELINES Version April 20, 2017 1. Introduction The goals of the CARRA Publication and Presentation Guidelines are to: a) Promote timely and high-quality presentation
CADTH HEALTH TECHNOLOGY MANAGEMENT PROGRAM Horizon Scanning Products and Services Processes
 CADTH HEALTH TECHNOLOGY MANAGEMENT PROGRAM Horizon Scanning Products and Services Processes Service Line: Health Technology Management Program Version: 1.0 Publication Date: September 2017 Report Length:
CADTH HEALTH TECHNOLOGY MANAGEMENT PROGRAM Horizon Scanning Products and Services Processes Service Line: Health Technology Management Program Version: 1.0 Publication Date: September 2017 Report Length:
Investigation i of potentially contaminated sites - Code of Practice. Richard Owen Ove Arup & Partners Limited
 The proposed changes to BS10175 Investigation i of potentially contaminated sites - Code of Practice Richard Owen Ove Arup & Partners Limited CONTENT OF THE PRESENTATION Background and Objectives Revision
The proposed changes to BS10175 Investigation i of potentially contaminated sites - Code of Practice Richard Owen Ove Arup & Partners Limited CONTENT OF THE PRESENTATION Background and Objectives Revision
Issues in Emerging Health Technologies Bulletin Process
 Issues in Emerging Health Technologies Bulletin Process Updated: April 2015 Version 1.0 REVISION HISTORY Periodically, this document will be revised as part of ongoing process improvement activities. The
Issues in Emerging Health Technologies Bulletin Process Updated: April 2015 Version 1.0 REVISION HISTORY Periodically, this document will be revised as part of ongoing process improvement activities. The
***************************************************************************** DRAFT UFGS- 01 XX XX (FEB 2014)
 DRAFT UFGS- 01 XX XX (FEB 2014) ------------------------ Drafting Activity: USACE UNIFIED FACILITIES GUIDE SPECIFICATION SECTION TABLE OF CONTENTS DIVISION 01 GENERAL REQUIREMENTS SECTION 01 XX XX (FEB
DRAFT UFGS- 01 XX XX (FEB 2014) ------------------------ Drafting Activity: USACE UNIFIED FACILITIES GUIDE SPECIFICATION SECTION TABLE OF CONTENTS DIVISION 01 GENERAL REQUIREMENTS SECTION 01 XX XX (FEB
CPC Essentials I Part A Introduction to CPC Essentials and Patent Classification Systems
 CPC Essentials I Part A Introduction to CPC Essentials and Patent Classification Systems Classification Quality and International Cooperation (CQIC) Division Office of International Patent Cooperation
CPC Essentials I Part A Introduction to CPC Essentials and Patent Classification Systems Classification Quality and International Cooperation (CQIC) Division Office of International Patent Cooperation
Exploring emerging ICT-enabled governance models in European cities
 Exploring emerging ICT-enabled governance models in European cities EXPGOV Project Research Plan D.1 - FINAL (V.2.0, 27.01.2009) This document has been drafted by Gianluca Misuraca, Scientific Officer
Exploring emerging ICT-enabled governance models in European cities EXPGOV Project Research Plan D.1 - FINAL (V.2.0, 27.01.2009) This document has been drafted by Gianluca Misuraca, Scientific Officer
Title: LE Task Group Report - Session #45
 Title: LE Task Group Report - Session #45 Document Number: IEEE 802.16h-06/025r1 Date Submitted: September 28, 2006 Source: Chair of LE TG: Mariana Goldhamer Voice:+972 3 645 6241 mariana.goldhamer@alvarion.com
Title: LE Task Group Report - Session #45 Document Number: IEEE 802.16h-06/025r1 Date Submitted: September 28, 2006 Source: Chair of LE TG: Mariana Goldhamer Voice:+972 3 645 6241 mariana.goldhamer@alvarion.com
Quality Regulation under Revised Pharmaceutical Affair Law
 Quality Regulation under Revised Pharmaceutical Affair Law Yukio Hiyama, Ph.D. Division of Drugs, National Institute of Health Sciences, the Ministry of Health, Labour and Welfare, Japan E mail hiyama@nihs.go.jp
Quality Regulation under Revised Pharmaceutical Affair Law Yukio Hiyama, Ph.D. Division of Drugs, National Institute of Health Sciences, the Ministry of Health, Labour and Welfare, Japan E mail hiyama@nihs.go.jp
Mesh Networks in Fixed Broadband Wireless Access
 Mesh Networks in Fixed Broadband Wireless Access IEEE 802.16 Presentation Submission Template (Rev. 8.3) Document Number: IEEE C802.16-03/10r1 Date Submitted: 2003-07-21 Source: Barry Lewis Voice: +44
Mesh Networks in Fixed Broadband Wireless Access IEEE 802.16 Presentation Submission Template (Rev. 8.3) Document Number: IEEE C802.16-03/10r1 Date Submitted: 2003-07-21 Source: Barry Lewis Voice: +44
Joint Industry Programme on E&P Sound and Marine Life - Phase III
 Joint Industry Programme on E&P Sound and Marine Life - Phase III Request for Proposals Number: JIP III-15-03 Long Term Fixed Acoustic Monitoring of Marine Mammals throughout the Life Cycle of an Offshore
Joint Industry Programme on E&P Sound and Marine Life - Phase III Request for Proposals Number: JIP III-15-03 Long Term Fixed Acoustic Monitoring of Marine Mammals throughout the Life Cycle of an Offshore
A Brief Introduction to the Regulatory Environment of Medical Device Supervision. CFDA Department of Legal Affairs Liu Pei
 A Brief Introduction to the Regulatory Environment of Medical Device Supervision CFDA Department of Legal Affairs Liu Pei Development Trend of Medical Device Industry Development Opportunities of Medical
A Brief Introduction to the Regulatory Environment of Medical Device Supervision CFDA Department of Legal Affairs Liu Pei Development Trend of Medical Device Industry Development Opportunities of Medical
TGA Discussion Paper 3D Printing Technology in the Medical Device Field Australian Regulatory Considerations
 TGA Discussion Paper 3D Printing Technology in the Medical Device Field Australian Regulatory Considerations MTAA Response - October 2017 October 2017 Australian Regulatory Considerations Page 1 of 7 Level
TGA Discussion Paper 3D Printing Technology in the Medical Device Field Australian Regulatory Considerations MTAA Response - October 2017 October 2017 Australian Regulatory Considerations Page 1 of 7 Level
Village of Glenview Appearance Commission
 Village of Glenview Appearance Commission STAFF REPORT June 13, 2018 TO: Chairman and Appearance Commissioners FROM: Community Development Department CASE #: A2018-062 LOCATION: PROJECT NAME: 1533 Waukegan
Village of Glenview Appearance Commission STAFF REPORT June 13, 2018 TO: Chairman and Appearance Commissioners FROM: Community Development Department CASE #: A2018-062 LOCATION: PROJECT NAME: 1533 Waukegan
Transmission Availability Data System Phase II Final Report
 Transmission Availability Data System Phase II Final Report Prepared by the Transmission Availability Data System Task Force for the NERC Planning Committee Approved by the Planning Committee on: Table
Transmission Availability Data System Phase II Final Report Prepared by the Transmission Availability Data System Task Force for the NERC Planning Committee Approved by the Planning Committee on: Table
IAB Europe Guidance THE DEFINITION OF PERSONAL DATA. IAB Europe GDPR Implementation Working Group WHITE PAPER
 IAB Europe Guidance WHITE PAPER THE DEFINITION OF PERSONAL DATA Five Practical Steps to help companies comply with the E-Privacy Working Directive Paper 02/2017 IAB Europe GDPR Implementation Working Group
IAB Europe Guidance WHITE PAPER THE DEFINITION OF PERSONAL DATA Five Practical Steps to help companies comply with the E-Privacy Working Directive Paper 02/2017 IAB Europe GDPR Implementation Working Group
Information points report
 Information points report ESCO (2017) SEC 004 FINAL Document Date: 09/02/2017 Last update: 08/03/2017 Table of Contents Table of Contents... 2 Purpose of this document... 3 Third meeting of the Member
Information points report ESCO (2017) SEC 004 FINAL Document Date: 09/02/2017 Last update: 08/03/2017 Table of Contents Table of Contents... 2 Purpose of this document... 3 Third meeting of the Member
PMDA perspective on Quality by Design for pharmaceutical products
 PMDA perspective on Quality by Design for pharmaceutical products Junichi Fukuchi, Ph.D. Office of Cellular and Tissue-based Products Pharmaceuticals and Medical Devices Agency (PMDA) Annual conference
PMDA perspective on Quality by Design for pharmaceutical products Junichi Fukuchi, Ph.D. Office of Cellular and Tissue-based Products Pharmaceuticals and Medical Devices Agency (PMDA) Annual conference
The Second Health Information Technology Summit
 The Second Health Information Technology Summit Shared HIT/HIPAA Issues: The National Provider Identifier and the Impact on Payers, Health Plans and Clearinghouses Session 5.05 Tom Polhemus Principal Operations
The Second Health Information Technology Summit Shared HIT/HIPAA Issues: The National Provider Identifier and the Impact on Payers, Health Plans and Clearinghouses Session 5.05 Tom Polhemus Principal Operations
COST European Cooperation in Science and Technology
 COST European Cooperation in Science and Technology Introduction to the COST Framework Programme COST is supported by the EU Framework Programme ESF provides the COST Office through a European Commission
COST European Cooperation in Science and Technology Introduction to the COST Framework Programme COST is supported by the EU Framework Programme ESF provides the COST Office through a European Commission
European Workgroup. 7th May 2015
 European Workgroup Place your chosen image here. The four corners must just cover the arrow tips. For covers, the three pictures should be the same size and in a straight line. 7th May 2015 Code Status
European Workgroup Place your chosen image here. The four corners must just cover the arrow tips. For covers, the three pictures should be the same size and in a straight line. 7th May 2015 Code Status
3. How to prepare a successful proposal?
 3. How to prepare a successful proposal? COST is supported by the EU Framework Programme 44 ESF provides the COST Office through a European Commission contract COST Open Call Official publication (incl.the
3. How to prepare a successful proposal? COST is supported by the EU Framework Programme 44 ESF provides the COST Office through a European Commission contract COST Open Call Official publication (incl.the
ATMF revision. Agenda item 8 of the 25 th Revision Committee OTIF
 ORGANISATION INTERGOUVERNEMENTALE POUR LES TRANSPORTS INTERNATIONAUX FERROVIAIRES ZWISCHENSTAATLICHE ORGANISATION FÜR DEN INTERNATIONALEN EISENBAHNVERKEHR INTERGOVERNMENTAL ORGANISATION FOR INTERNATIONAL
ORGANISATION INTERGOUVERNEMENTALE POUR LES TRANSPORTS INTERNATIONAUX FERROVIAIRES ZWISCHENSTAATLICHE ORGANISATION FÜR DEN INTERNATIONALEN EISENBAHNVERKEHR INTERGOVERNMENTAL ORGANISATION FOR INTERNATIONAL
STATEMENT OF WORK Environmental Assessment for the Red Cliffs/Long Valley Land Exchange in Washington County, Utah
 I. Introduction STATEMENT OF WORK Environmental Assessment for the Red Cliffs/Long Valley Land Exchange in Washington County, Utah The Bureau of Land Management s (BLM) St. George Field Office (SGFO) requires
I. Introduction STATEMENT OF WORK Environmental Assessment for the Red Cliffs/Long Valley Land Exchange in Washington County, Utah The Bureau of Land Management s (BLM) St. George Field Office (SGFO) requires
CDISC Intrachange Silver Spring German User Group Meeting, 24-Sep-2013 Melanie Füllbeck
 CDISC Intrachange 2013 30.07. - 01.08.2013 Silver Spring German User Group Meeting, 24-Sep-2013 Melanie Füllbeck Intrachange Agenda 30.07.2013 - Technical Plan, Roadmap - Study Data Standards at FDA -
CDISC Intrachange 2013 30.07. - 01.08.2013 Silver Spring German User Group Meeting, 24-Sep-2013 Melanie Füllbeck Intrachange Agenda 30.07.2013 - Technical Plan, Roadmap - Study Data Standards at FDA -
E5 Implementation Working Group Questions & Answers (R1) Current version dated June 2, 2006
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE E5 Implementation Working Group & (R1) Current version dated June 2, 2006 ICH Secretariat,
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE E5 Implementation Working Group & (R1) Current version dated June 2, 2006 ICH Secretariat,
Massport Capital Programs Enterprise Schedule Management. Primavera Schedule Toolkit Design. January 2018 Version 1.0
 Massport Capital Programs Enterprise Schedule Management Primavera Schedule Toolkit Design January 2018 Version 1.0 Contents 1.0 INTRODUCTION 2 1.1 DISCLAIMER 3 2.0 TOOLKIT SCHEDULE COMPONENTS 4 2.1 SCHEDULE
Massport Capital Programs Enterprise Schedule Management Primavera Schedule Toolkit Design January 2018 Version 1.0 Contents 1.0 INTRODUCTION 2 1.1 DISCLAIMER 3 2.0 TOOLKIT SCHEDULE COMPONENTS 4 2.1 SCHEDULE
Convergence and Differentiation within the Framework of European Scientific and Technical Cooperation on HTA
 EUnetHTA European network for Health Technology Assessment Convergence and Differentiation within the Framework of European Scientific and Technical Cooperation on HTA University of Tokyo, October 24,
EUnetHTA European network for Health Technology Assessment Convergence and Differentiation within the Framework of European Scientific and Technical Cooperation on HTA University of Tokyo, October 24,
Combined Air Emissions Reporting System (CAER) A State s Perspective
 Combined Air Emissions Reporting System (CAER) A State s Perspective Kathy Pendleton, P.E. Air Quality Division Texas Commission on Environmental Quality 2016 Midwest and Central States Air Quality Workshop
Combined Air Emissions Reporting System (CAER) A State s Perspective Kathy Pendleton, P.E. Air Quality Division Texas Commission on Environmental Quality 2016 Midwest and Central States Air Quality Workshop
Towards an MDA-based development methodology 1
 Towards an MDA-based development methodology 1 Anastasius Gavras 1, Mariano Belaunde 2, Luís Ferreira Pires 3, João Paulo A. Almeida 3 1 Eurescom GmbH, 2 France Télécom R&D, 3 University of Twente 1 gavras@eurescom.de,
Towards an MDA-based development methodology 1 Anastasius Gavras 1, Mariano Belaunde 2, Luís Ferreira Pires 3, João Paulo A. Almeida 3 1 Eurescom GmbH, 2 France Télécom R&D, 3 University of Twente 1 gavras@eurescom.de,
ASX SETTLEMENT PROCEDURE GUIDELINES
 SECTION 11: CORPORATE ACTION PRINCIPLES... 11-1 11.1 CORPORATE ACTION CONCEPTS... 11-1 11.1.1 The Ex-Period... 11-1 11.1.2 Basis of Movement... 11-2 11.1.3 Deferred Settlement... 11-5 11.1.4 Subregister
SECTION 11: CORPORATE ACTION PRINCIPLES... 11-1 11.1 CORPORATE ACTION CONCEPTS... 11-1 11.1.1 The Ex-Period... 11-1 11.1.2 Basis of Movement... 11-2 11.1.3 Deferred Settlement... 11-5 11.1.4 Subregister
CASE REPORT FORM DESIGN AND IMPLEMENTATION
 CASE REPORT FORM DESIGN AND IMPLEMENTATION DOCUMENT NO.: CR013 v1.0 AUTHOR: Elizabeth Craig ISSUE DATE: 25 October 2016 EFFECTIVE DATE: 08 November 2016 1 INTRODUCTION 1.1 The Academic & Clinical Central
CASE REPORT FORM DESIGN AND IMPLEMENTATION DOCUMENT NO.: CR013 v1.0 AUTHOR: Elizabeth Craig ISSUE DATE: 25 October 2016 EFFECTIVE DATE: 08 November 2016 1 INTRODUCTION 1.1 The Academic & Clinical Central
Establishment of Electrical Safety Regulations Governing Generation, Transmission and Distribution of Electricity in Ontario
 August 7, 2001 See Distribution List RE: Establishment of Electrical Safety Regulations Governing Generation, Transmission and Distribution of Electricity in Ontario Dear Sir/Madam: The Electrical Safety
August 7, 2001 See Distribution List RE: Establishment of Electrical Safety Regulations Governing Generation, Transmission and Distribution of Electricity in Ontario Dear Sir/Madam: The Electrical Safety
Definition of Bulk Electric System Phase 2
 Definition of Bulk Electric System Phase 2 NERC Industry Webinar Peter Heidrich, FRCC, Standard Drafting Team Chair June 26, 2013 Topics Phase 2 - Definition of Bulk Electric System (BES) Project Order
Definition of Bulk Electric System Phase 2 NERC Industry Webinar Peter Heidrich, FRCC, Standard Drafting Team Chair June 26, 2013 Topics Phase 2 - Definition of Bulk Electric System (BES) Project Order
Load-Frequency Control and Reserves Network Code. David Bunney JESG 19 March 2013
 Load-Frequency Control and Reserves Network Code David Bunney JESG 19 March 2013 Agenda Overview and Timescales Stakeholder Engagement Overview of the Code More detailed discussion on Frequency Quality
Load-Frequency Control and Reserves Network Code David Bunney JESG 19 March 2013 Agenda Overview and Timescales Stakeholder Engagement Overview of the Code More detailed discussion on Frequency Quality
Office for Nuclear Regulation
 Office for Nuclear Regulation Redgrave Court Merton Road Bootle Merseyside L20 7HS www.hse.gov.uk/nuclear PROJECT ASSESSMENT REPORT Report Identifier: ONR-Policy-all-PAR-11-001 Revision: 2 Project: Implementation
Office for Nuclear Regulation Redgrave Court Merton Road Bootle Merseyside L20 7HS www.hse.gov.uk/nuclear PROJECT ASSESSMENT REPORT Report Identifier: ONR-Policy-all-PAR-11-001 Revision: 2 Project: Implementation
VAR Voltage and Reactive Control. A. Introduction
 VAR-001-5 Voltage and Reactive Control A. Introduction 1. Title: Voltage and Reactive Control 2. Number: VAR-001-5 3. Purpose: To ensure that voltage levels, reactive flows, and reactive resources are
VAR-001-5 Voltage and Reactive Control A. Introduction 1. Title: Voltage and Reactive Control 2. Number: VAR-001-5 3. Purpose: To ensure that voltage levels, reactive flows, and reactive resources are
Cloud Based Plan Review Process (Applicant)
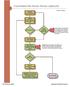 1 Cloud Based Plan Review Process (Applicant) Organize Documentation Process Overview Upload Documents To Mercer Island FTP Site No Initial Submittal? Yes Intake Screening Approved? CST will inform you
1 Cloud Based Plan Review Process (Applicant) Organize Documentation Process Overview Upload Documents To Mercer Island FTP Site No Initial Submittal? Yes Intake Screening Approved? CST will inform you
.2 Accompany all submissions with a transmittal letter, in duplicate, containing:.4 Specification Section number for each submittal
 City of Winnipeg Brady Road Landfill Site Section 01300 New Entrance and Scale Facility Page 1 of 4 SUBMITTALS 1. SHOP DRAWINGS 1.1 General.1 Arrange for the preparation of clearly identified Shop Drawings
City of Winnipeg Brady Road Landfill Site Section 01300 New Entrance and Scale Facility Page 1 of 4 SUBMITTALS 1. SHOP DRAWINGS 1.1 General.1 Arrange for the preparation of clearly identified Shop Drawings
Approaches and tools to facilitate CSA and ES development
 Approaches and tools to facilitate CSA and ES development REACH Implementation Workshop, Brussels, 7 December 2012 Dook Noij, Frank Schnoeder, Mercedes Viñas Dow / DuPont / Cefic On behalf of the Cefic
Approaches and tools to facilitate CSA and ES development REACH Implementation Workshop, Brussels, 7 December 2012 Dook Noij, Frank Schnoeder, Mercedes Viñas Dow / DuPont / Cefic On behalf of the Cefic
Academic Medical Center Security and Privacy Conference (AMCSP)
 Academic Medical Center Security and Privacy Conference (AMCSP) econsent Guidance and Use Cases JUNE 27, 2016 Colleen Lawrence, PhD, CCRP, Research Services Consultant, Vanderbilt University Medical Center
Academic Medical Center Security and Privacy Conference (AMCSP) econsent Guidance and Use Cases JUNE 27, 2016 Colleen Lawrence, PhD, CCRP, Research Services Consultant, Vanderbilt University Medical Center
ASX CHESS Replacement Project Webinar
 ASX CHESS Replacement Project Webinar Q4 update 14 December 2017 Audio Trouble Shooting & Reminder to Submit Questions Your Participation Having trouble hearing via Computer Audio? Switch to Phone call!
ASX CHESS Replacement Project Webinar Q4 update 14 December 2017 Audio Trouble Shooting & Reminder to Submit Questions Your Participation Having trouble hearing via Computer Audio? Switch to Phone call!
Inter-Association Task Force
 Inter-Association Task Force Presentation to EMA Workshop Prevention of Drug Shortages Based on Quality and Manufacturing Issues 9 th October 2015 1 Topics for Today Background and history of task force
Inter-Association Task Force Presentation to EMA Workshop Prevention of Drug Shortages Based on Quality and Manufacturing Issues 9 th October 2015 1 Topics for Today Background and history of task force
Claus Mortensen, Medicines Inspector. Danish Medicines Agency. Member of the EMEA PAT team
 Claus Mortensen, Medicines Inspector Danish Medicines Agency Member of the EMEA PAT team EMEA progress and status what is needed to document scientific understanding in a PAT application EMEA PAT team
Claus Mortensen, Medicines Inspector Danish Medicines Agency Member of the EMEA PAT team EMEA progress and status what is needed to document scientific understanding in a PAT application EMEA PAT team
Frequently Asked Questions
 Table of Contents Who should an Investigator contact to submit an ERP proposal? What type of information is needed for Medtronic to review? Is a protocol required? Why is so much information necessary?
Table of Contents Who should an Investigator contact to submit an ERP proposal? What type of information is needed for Medtronic to review? Is a protocol required? Why is so much information necessary?
Japan s FinTech Vision
 Japan s FinTech Vision First Comprehensive Industrial Finance Division Economic and Industrial Policy Bureau Ministry of Economy, Trade and Industry 1 FinTech: New Finance to Support the Fourth Industrial
Japan s FinTech Vision First Comprehensive Industrial Finance Division Economic and Industrial Policy Bureau Ministry of Economy, Trade and Industry 1 FinTech: New Finance to Support the Fourth Industrial
RESEARCH AND INNOVATION STRATEGY. ANZPAA National Institute of Forensic Science
 RESEARCH AND INNOVATION STRATEGY ANZPAA National Institute of Forensic Science 2017-2020 0 CONTENTS INTRODUCTION... 3 PURPOSE... 4 STRATEGY FOUNDATION... 5 NEW METHODS AND TECHNOLOGY... 5 ESTABLISHED METHODS
RESEARCH AND INNOVATION STRATEGY ANZPAA National Institute of Forensic Science 2017-2020 0 CONTENTS INTRODUCTION... 3 PURPOSE... 4 STRATEGY FOUNDATION... 5 NEW METHODS AND TECHNOLOGY... 5 ESTABLISHED METHODS
(R) Aerospace First Article Inspection Requirement FOREWORD
 AEROSPACE STANDARD AS9102 Technically equivalent to AECMA pren 9102 Issued 2000-08 Revised 2004-01 REV. A Supersedes AS9012 (R) Aerospace First Article Inspection Requirement FOREWORD In December 1998,
AEROSPACE STANDARD AS9102 Technically equivalent to AECMA pren 9102 Issued 2000-08 Revised 2004-01 REV. A Supersedes AS9012 (R) Aerospace First Article Inspection Requirement FOREWORD In December 1998,
IEEE Broadband Wireless Access Working Group < Proposed PAR to convert P802.16d from Amendment to Revision
 2003-05-15 IEEE C802.16-03/08 Project Title Date Submitted IEEE 802.16 Broadband Wireless Access Working Group Proposed PAR to convert P802.16d from Amendment to Revision 2003-05-15
2003-05-15 IEEE C802.16-03/08 Project Title Date Submitted IEEE 802.16 Broadband Wireless Access Working Group Proposed PAR to convert P802.16d from Amendment to Revision 2003-05-15
IEEE C802.16h-06/071. IEEE Broadband Wireless Access Working Group <
 Project Title Date Submitted IEEE 802.16 Broadband Wireless Access Working Group P802.16h Working Document structure clarification 2006-09-17 Source(s) Paul Piggin NextWave Broadband
Project Title Date Submitted IEEE 802.16 Broadband Wireless Access Working Group P802.16h Working Document structure clarification 2006-09-17 Source(s) Paul Piggin NextWave Broadband
Thank you for the opportunity to comment on the Audit Review and Compliance Branch s (ARC) recent changes to its auditing procedures.
 Jim Riva, Chief Audit Review and Compliance Branch Agricultural Marketing Service United States Department of Agriculture 100 Riverside Parkway, Suite 135 Fredericksburg, VA 22406 Comments sent to: ARCBranch@ams.usda.gov
Jim Riva, Chief Audit Review and Compliance Branch Agricultural Marketing Service United States Department of Agriculture 100 Riverside Parkway, Suite 135 Fredericksburg, VA 22406 Comments sent to: ARCBranch@ams.usda.gov
MISSISSIPPI STATE UNIVERSITY Office of Planning Design and Construction Administration
 SECTION 01 340 - SHOP DRAWINGS, PRODUCT DATA AND SAMPLES PART 1 - GENERAL 1.1 RELATED DOCUMENTS A. Drawings and general provisions of the Contract, including General and Supplementary Conditions and other
SECTION 01 340 - SHOP DRAWINGS, PRODUCT DATA AND SAMPLES PART 1 - GENERAL 1.1 RELATED DOCUMENTS A. Drawings and general provisions of the Contract, including General and Supplementary Conditions and other
Ohio Department of Transportation Division of Production Management Office of Geotechnical Engineering. Geotechnical Bulletin
 Ohio Department of Transportation Division of Production Management Office of Geotechnical Engineering Geotechnical Bulletin GB 5 GEOTECHNICAL SUBMISSION GUIDELINES Geotechnical Bulletin GB5 was developed
Ohio Department of Transportation Division of Production Management Office of Geotechnical Engineering Geotechnical Bulletin GB 5 GEOTECHNICAL SUBMISSION GUIDELINES Geotechnical Bulletin GB5 was developed
BIM Policy Development: Different Countries, Common Approaches
 : Different Countries, Common Approaches Bilal Succar, PhD Director, BIMexcellence.com Mohamad Kassem, PhD Senior Lecturer + Enterprise Fellow, Teesside University 2 this presentation is in Two Parts:
: Different Countries, Common Approaches Bilal Succar, PhD Director, BIMexcellence.com Mohamad Kassem, PhD Senior Lecturer + Enterprise Fellow, Teesside University 2 this presentation is in Two Parts:
UDW Technology Conference Dan McLeod / John Jacobson Lockheed Martin MS2 July 27, Secure Energy for America
 RPSEA 09121-3300 3300-05 05 Autonomous Inspection of Subsea Facilities Phase I Final Presentation / Phase II Status Report UDW Technology Conference Dan McLeod / John Jacobson Lockheed Martin MS2 July
RPSEA 09121-3300 3300-05 05 Autonomous Inspection of Subsea Facilities Phase I Final Presentation / Phase II Status Report UDW Technology Conference Dan McLeod / John Jacobson Lockheed Martin MS2 July
Recreation Facility Hours
 NORMAL HOURS Recreation Facility Hours August 1, 2016 July 31, 2017 Last Revised: July 29, 2016 Effective Dates 2016: AUG 23 31, SEP 1 2, SEP 6 16, SEP 18 23, SEP 25 30, OCT 1 7, OCT 9 18, OCT 24 30, NOV
NORMAL HOURS Recreation Facility Hours August 1, 2016 July 31, 2017 Last Revised: July 29, 2016 Effective Dates 2016: AUG 23 31, SEP 1 2, SEP 6 16, SEP 18 23, SEP 25 30, OCT 1 7, OCT 9 18, OCT 24 30, NOV
Preparation of requirements. Part I Notification principles and time schedule
 ASBU ITU Workshop 2004 22 25 November 2004 Damascus, Syria Preparation of requirements for T-DAB T and DVB-T Part I Notification principles and time schedule B. Rackov, Radiocommunication Bureau Notification
ASBU ITU Workshop 2004 22 25 November 2004 Damascus, Syria Preparation of requirements for T-DAB T and DVB-T Part I Notification principles and time schedule B. Rackov, Radiocommunication Bureau Notification
Intimate Communications Hub Interface Specification Report to Secretary of State
 Intimate Communications Hub Interface Specification Report to Secretary of State DCC V1.0 28/02/14 Page 1 of 14 Executive Summary 1. DCC is required in accordance with the terms of its Licence to produce,
Intimate Communications Hub Interface Specification Report to Secretary of State DCC V1.0 28/02/14 Page 1 of 14 Executive Summary 1. DCC is required in accordance with the terms of its Licence to produce,
April 2015 newsletter. Efficient Energy Planning #3
 STEEP (Systems Thinking for Efficient Energy Planning) is an innovative European project delivered in a partnership between the three cities of San Sebastian (Spain), Bristol (UK) and Florence (Italy).
STEEP (Systems Thinking for Efficient Energy Planning) is an innovative European project delivered in a partnership between the three cities of San Sebastian (Spain), Bristol (UK) and Florence (Italy).
Consolidation of Navigation Safety Regulations IMO - NCSR / MSC Updates
 Mariners Workshop - January 23 th and 24 th 2019: Consolidation of Navigation Safety Regulations IMO - NCSR / MSC Updates IMO UPDATE NCSR / MSC Sessions Outcome of the Navigation, Communications and Search
Mariners Workshop - January 23 th and 24 th 2019: Consolidation of Navigation Safety Regulations IMO - NCSR / MSC Updates IMO UPDATE NCSR / MSC Sessions Outcome of the Navigation, Communications and Search
Phase 1 US Compliance Report
 Implementation of Regulatory Information Submission Standards (IRISS) ectd Tool Interoperability Group (ETIG) ectd Tool Interoperability and Compliance Study 3 (ETICS 3) ETICS 15 April 2011 Implementation
Implementation of Regulatory Information Submission Standards (IRISS) ectd Tool Interoperability Group (ETIG) ectd Tool Interoperability and Compliance Study 3 (ETICS 3) ETICS 15 April 2011 Implementation
Next Generation Mobile Networks
 Title: NGMN liaison response on invitation to update the information in the IMT2020 roadmap Source: NGMN Office To: ITU-T JCA-IMT2020 CC: Date: 24 th October 2017 Contacts: Klaus Moschner (klaus.moschner@ngmn.org)
Title: NGMN liaison response on invitation to update the information in the IMT2020 roadmap Source: NGMN Office To: ITU-T JCA-IMT2020 CC: Date: 24 th October 2017 Contacts: Klaus Moschner (klaus.moschner@ngmn.org)
Value Paper. Are you PAT and QbD Ready? Get up to speed
 Value Paper Are you PAT and QbD Ready? Get up to speed PAT and Quality-by-Design As PAT and Quality -by-design (QbD) become an integral part of the regulatory framework, automation group ABB argues more
Value Paper Are you PAT and QbD Ready? Get up to speed PAT and Quality-by-Design As PAT and Quality -by-design (QbD) become an integral part of the regulatory framework, automation group ABB argues more
Village of Glenview Appearance Commission
 Village of Glenview Appearance Commission STAFF REPORT July 26, 2017 TO: Chairman and Appearance Commissioners FROM: Community Development Department CASE #: A2017-064 LOCATION: PROJECT NAME: 3534 Milwaukee
Village of Glenview Appearance Commission STAFF REPORT July 26, 2017 TO: Chairman and Appearance Commissioners FROM: Community Development Department CASE #: A2017-064 LOCATION: PROJECT NAME: 3534 Milwaukee
ediscovery and Digital Evidence Online Course
 ediscovery and Digital Evidence Online Course The Convergence Between Law & Technology Instructor: Michael R. Arkfeld Dates February 26, 2014 to April 16, 2014 Day and Time of Online Sessions Wednesday
ediscovery and Digital Evidence Online Course The Convergence Between Law & Technology Instructor: Michael R. Arkfeld Dates February 26, 2014 to April 16, 2014 Day and Time of Online Sessions Wednesday
Lorenza Jachia Secretary, Working Party on Regulatory Cooperation and Standardization Policies, UN Economic Commission for Europe
 The UNECE Sectoral Initiative on Environments Equipment for Explosive A global legislative framework for Explosion Protection The comprehensive approach of the UNECE Model L Regulation Lorenza Jachia Secretary,
The UNECE Sectoral Initiative on Environments Equipment for Explosive A global legislative framework for Explosion Protection The comprehensive approach of the UNECE Model L Regulation Lorenza Jachia Secretary,
Exploiting the digital dividend a European approach: overview of the study for the European Commission
 Presentation for the Radio Spectrum Policy Group Exploiting the digital dividend a European approach: overview of the study for the European Commission Amit Nagpal, Lee Sanders, Richard Marsden, Gerry
Presentation for the Radio Spectrum Policy Group Exploiting the digital dividend a European approach: overview of the study for the European Commission Amit Nagpal, Lee Sanders, Richard Marsden, Gerry
Traditional Knowledge Digital Library. Presentation Adapted from Dr. V K Gupta, CSIR
 Traditional Knowledge Digital Library Presentation Adapted from Dr. V K Gupta, CSIR TRADITIONAL KNOWLEDGE (TK) UNDERSTANDING KNOWLEDGE IS THE FIRST STEP TO MANAGING IT EFFECTIVELY. Why Document? Know the
Traditional Knowledge Digital Library Presentation Adapted from Dr. V K Gupta, CSIR TRADITIONAL KNOWLEDGE (TK) UNDERSTANDING KNOWLEDGE IS THE FIRST STEP TO MANAGING IT EFFECTIVELY. Why Document? Know the
Procedure for Obtaining Verification of a Stormwater Manufactured Treatment Device from New Jersey Corporation for Advanced Technology
 Procedure for Obtaining Verification of a Stormwater Manufactured Treatment Device from New Jersey Corporation for Advanced Technology For use in accordance with the Stormwater Management Rules, N.J.A.C.
Procedure for Obtaining Verification of a Stormwater Manufactured Treatment Device from New Jersey Corporation for Advanced Technology For use in accordance with the Stormwater Management Rules, N.J.A.C.
Implementing Quality Systems
 Implementing Quality Systems CGMP By The Sea August 29, 2006 Chris Joneckis, Ph.D. Senior Advisor For CMC Issues Center For Biologics Evaluation And Research Add FDA Bar and Presentation Overview Driving
Implementing Quality Systems CGMP By The Sea August 29, 2006 Chris Joneckis, Ph.D. Senior Advisor For CMC Issues Center For Biologics Evaluation And Research Add FDA Bar and Presentation Overview Driving
DNV GL approval of service supplier scheme
 CLASS PROGRAMME DNVGL-CP-0484 Edition February 2016 The electronic pdf version of this document, available free of charge from http://www.dnvgl.com, is the officially binding version. FOREWORD DNV GL class
CLASS PROGRAMME DNVGL-CP-0484 Edition February 2016 The electronic pdf version of this document, available free of charge from http://www.dnvgl.com, is the officially binding version. FOREWORD DNV GL class
Submission of comments on Review of the Variations Guidelines (EC 1234/2008)
 12 July 2012 Submission of comments on Review of the Variations Guidelines (EC 1234/2008) Comments from: Name of organisation or individual Leem Please note that these comments and the identity of the
12 July 2012 Submission of comments on Review of the Variations Guidelines (EC 1234/2008) Comments from: Name of organisation or individual Leem Please note that these comments and the identity of the
WG food contact materials
 WG food contact materials Monday 30 January European Commission DG SANTE, Unit E2 Food Processing Technologies and Novel Foods Food Contact Materials This presentation does not present any official views
WG food contact materials Monday 30 January European Commission DG SANTE, Unit E2 Food Processing Technologies and Novel Foods Food Contact Materials This presentation does not present any official views
Agency Information Collection Activities; Proposed Collection; Comment Request; Good
 This document is scheduled to be published in the Federal Register on 06/12/2014 and available online at http://federalregister.gov/a/2014-13787, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
This document is scheduled to be published in the Federal Register on 06/12/2014 and available online at http://federalregister.gov/a/2014-13787, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
