Confocal Laser Scanning Microscope FV3000 FLUOVIEW. Next Generation FLUOVIEW for the Next Revolutions in Science
|
|
|
- Hilda Day
- 6 years ago
- Views:
Transcription
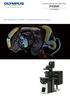
1 Confocal Laser Scanning Microscope FV3000 FLUOVIEW Next Generation FLUOVIEW for the Next Revolutions in Science
2 (1) (2) (3) 1
3 The FLUOVIEW FV3000 Series FV3000 and FV3000RS The Next Evolution of Confocal Laser Scanning Microscope Technology for Cell Biology, Cancer Research, Stem Cell Research, and Advanced Applications The FLUOVIEW FV3000 Series is designed to meet some of the most difficult challenges in modern science. With the high sensitivity and speed required for live cell and tissue imaging and the ease of use and flexibility required for microplate imaging and complex screening protocols, the FV3000 Series supports complete workflows from live cell 2D 6D (x,y,λ,z,t,p) imaging through image processing, like deconvolution, and analysis. Particular attention has been paid to the needs of cell biology (pages 5 6), cancer research (pages 7 8), and stem cell research (page 9). The FV3000 is optimized for macro to micro imaging of cells, tissues, and small organisms. With Olympus renowned optics at the heart of the system, the FV3000 features a new spectral detection concept for true multichannel spectral imaging with high sensitivity detection in multiple dynamic ranges so even dim signals can be separated. The optical path enables macro to micro imaging from 1.25X to 150X magnification combined with robust, intuitive automation to simplify complex experiments, including one-click cellsens macro analysis for cell counting and segmentation analysis. The precision of galvanometer scanning is combined with the speed of resonant scanning in the FV3000RS hybrid scanner so users can combine precision and high-speed imaging in one experiment. Built for long service life and low operating costs, the FV3000 uses long-lasting all diode lasers and LED illumination. The system features a modular, upgradable design that includes 2-tier detection options, easily upgradeable laser configurations, and the stable and flexible IX83 microscope with a field-upgradable z drift compensator (IX3-ZDC2) for fast and robust live cell autofocus. With user-savable and selectable software workflows, the system adjusts to individual needs. The facility manager tracking software makes it easy to track system usage by user, making the FV3000 the ideal confocal system for years of productive science in single and multi-user environments. 2
4 The FV3000 Series: Meeting the Challenges of Cell Biology, Cancer Research, Stem Cell Research, and Advanced Applications Cell Division, Proliferation, Counting, Cell Cycle, and Segmentation Analysis Cell proliferation is a key aspect of cancer research. The FV3000 has tools for imaging and measuring these critical events. (1) (2) (3) (4) Microfluidics and High-Speed Blood Flow Circulating tumor cells in peripheral blood and microfluidic device imaging can require high-speed imaging for accurate measurements. The FV3000RS provides high-speed imaging for critical velocity measurements to capture key events. (8) Silicone Objectives Optimized for Live Tissue Observation 3D imaging has become an increasingly important part of cancer research. Olympus exclusive silicone objectives provide clear and bright images at depth in live cells and tissues for accurate imaging and quantification. UPLSAPO60XS2 (NA 1.3, W.D. 0.3 mm, silicone oil ne = 1.4) 5 μm 5 μm 35 μm 35 μm UPLSAPO 60XO (NA 1.35, W.D mm, immersion oil ne = 1.52) (5) (6) Macro to Micro and Whole Slide Imaging Cell biology research demands the flexibility to image small organisms at the macro scale down to the micro at high resolution. The FV3000 Series features optics that enable macro to micro imaging for enhanced flexibility. Fast Calcium Dynamics Image calcium sparks and waves at speeds up to 438 frames per second. Slow heartbeats to visible rates and capture vast neuronal cell networks at full field of view at 30 frames per second. (9) (10) (11) (12) (13) (7) 3
5 Spheroid, Gel Matrix, Long-Term Time-Lapse, and Microplate Imaging Long-term time-lapse imaging of live cells in 3D captures physiologically relevant information. As stem cells grow into spheroids and organoids, the FV3000 Series enables precise, stable time-lapse imaging with high sensitivity and low phototoxicity. Super Resolution Olympus patented* confocal super resolution imaging provides an easy-to-use method for boosting resolution beyond the diffraction limit in fixed tissues. *US B/JP B FV-OSR (14) (15) (18) CONVENTIONAL CONFOCAL (16) Photoconversion and Stimulation Precise control of laser light stimulation timing and complex multipoint imaging and stimulation enable highly reproducible experiments for various studies. Spectral Unmixing Complex overlapping fluorescent protein spectra can complicate a range of biological studies. The FV3000 Series efficiently separates signals for accurate measurements and localization. (17) Intensity Time(μs) 4
6 Solutions for Cell Biology: Image Dynamic in vivo Processes in Large and Small Organisms with Very Low to High Magnification Macro to Micro and Whole Slide Imaging Cell biology requires high sensitivity, and deals with live organisms such as zebrafish and C. elegans. Large pieces of tissue and small organisms may require both high speeds as well as large fields of view to see the entire organism in context. Accurately imaging a large field of view requires precise automation and excellent optics. The FV3000 System is designed to image large tissues and small organisms with accurate stage control, image stitching, and an optical design that facilitates very low to high magnification (1.25X up to 150X). Since autofluorescence can be an issue for cell biologists, the FV3000 was designed to be a fully spectral system capable of highly sensitive and accurate spectral background, autofluorescence, and overlapping spectra (e.g. GFP/YFP) separation. 1.25X Objective Single Shot Acquisition with Blind Unmixing 1.25X Objective Single Shot Acquisition Mouse brain hemisection embedded for Expansion Microscopy (pre-expansion). Secondary antibody labels against GFP (Alexa Fluor 488, neurons), SV2 (Alexa Fluor 565, Red) Homer (Alexa Fluor 647, Blue). Sample courtesy of Dr. Ed Boyden and Dr. Fei Chen, MIT. Dendrite (anti-gfp Alexa Fluor 488, green) and synaptic marker (SV2, Alexa Fluor 565, red) Olympus Super Resolution image processed with cellsens advanced contrained iterative deconvolution. Average Full Width Half Maximum measurements ~135 nm. Image acquired with 100X 1.35 NA silicone objective. Sample courtesy of Dr. Ed Boyden and Dr. Fei Chen, MIT. 5
7 A new optical design means that even when using low magnification 30X silicone objectives with 1.05 NA, resolution can be boosted using Olympus super resolution technology FV-OSR. Silicone objectives also help provide low spherical aberration on tissues and small organisms, so object measurements and distances are accurate. The resonant scanner also helps reduce phototoxicity and photobleaching compared to regular galvo scanners by reducing triplet states of excited fluorophores and reactive oxygen species. Highly Dynamic Imaging Small organisms are often favored as models for studying dynamic in vivo processes, so the FV3000RS is equipped with a very accurate resonant scanner, facilitating applications such as studying a beating heart, blood flow, calcium signaling, and other dynamic events at up to 438 frames per second. With the FV3000RS, switching between the high-precision galvanometer and high-speed resonance scanner is as simple as a mouse click. The resonance scanner maintains the same field of view so users won't get lost when switching between high-speed and high-precision scanning. Resonance images undergo post-processing with rolling average filtering for time gate image averaging while improving signal-to-noise. Ratio imaging can employ an Intensity Modulated Display (IMD) so real signal stands out above background noise. Selecting the spectral range is simple, and spectral unmixing is fast and automated. Intensity Modulated Display of CFP/YFP ratio result during spontaneous contractions of in vitro cardiomyocyte. Image data courtesy of Yusuke Nino and Atsushi Miyawaki, Cell Function Dynamics, Brain Science Institute of RIKEN. 6
8 Solutions for Cancer Research: Accurate 3D Cell and Tissue Imaging, High-Speed Blood Flow, Microfluidic Imaging, and Robust Analysis Cell Division, Proliferation, Counting, Cell Cycle, and Segmentation Analysis The FV3000 Series incorporates the range of technologies necessary for cancer research imaging studies. In live cell cancer studies, sensitive fluorescence detection, optimized optics, and analytical tools such as cell counting and segmentation analysis are essential. With the emergence of microfluidics and a focus on circulating tumor cells, high-speed acquisitions can make the difference between success and failure in an experiment. Accuracy and repeatability are equally important; cell cycle checkpoint times must be reliably tracked, 3D images of cells must correctly represent their shape and size, and images need to be bright and clear for segmentation analysis. Olympus silicone objectives are optimized for tissue imaging. The FV3000 Series high-sensitivity cooled GaAsP detection unit with high signal-to-noise galvo and resonant scanning and robust software make imaging accurate and reproducible for reliable results. Platelets bound to thrombosis in blood vessel of mouse. Images taken 30 fps in full frame by resonant scanner with 2 CH GaAsP PMTs. Image data courtesy of Dr. Takuya Hiratsuka, Dr. Michiyuki Matsuda, Graduate School of Biostudies, Kyoto University. NK-cell mediated cell killing after therapeutic anitbody application (blue). GFP labeled NK-cells (green). DAPI uptake marking dead cells (Red). Image data courtesy of Dr. Yuji Mishima, Cancer Chemotherapy Center, Japanese Foundation for Cancer Research. 5 μm 5 μm 35 μm 35 μm UPLSAPO60XS2 (NA 1.3, W.D. 0.3 mm, silicone oil ne = 1.4) UPLSAPO 60XO (NA 1.35, W.D mm, immersion oil ne = 1.52) ScaleA2-treated neocortex Image data courtesy of Motokazu Uchigashima, M.D., Ph.D., Masahiko Watanabe, M.D., Ph.D., Departments of Anatomy, Hokkaido University Graduate School of Medicine. 7
9 The system s sensitivity coupled with the laser power monitor and two freely selectable ranges for laser power help provide that apoptosis is part of the experiment and not caused by phototoxicity. The spectral sensitivity and accuracy enable researchers to conduct multi-color fluorescence labeling experiments with multiple biomarkers. Complex Tasks Made Simple Cancer research is complex but measuring proliferation with the FV3000 isn't. With cellsens macro capabilities, time-lapse images can be processed and counted and reports generated with a single mouse click. The layout of the acquisition software can be customized according to specific applications and immediately selected on startup, making workflows logical and tailored to a customer's needs. Specific experiment conditions can easily be reloaded, taking the guess work out of reproducing results. Fucci cell cycle counting and expansion by cellsens. Image data courtesy of Atsushi Miyawaki, Cell Function Dynamics, Brain Science Institute of RIKEN. 3D Time-lapse of mouse embryonic fibroblast labeled with silicone rhodamine docetaxol (Tubulin), imaged with 100X silicone objective and 30 fps resonant scanning followed by cellsens deconvolution. Image data courtesy of Dr. Markus Delling, Harvard University. 512 x 512 pixel A 0min B 512 x 32 pixel 512 x 512 pixel Scanning size Interval time 10min 20min 30min 30m30s Every 10 minutes 0 min 41m00s 49m30s 59m30s 60min Every 30 seconds 30 min 65min 70min 75min 80min 85min 90min Every 5 minutes 60 min 90 min Sequence Manager allows for variable time-lapse 8
10 Solutions for Stem Cell Imaging: Z Drift Compensator, and Intuitive Software for Accurate Long-Term and Multipoint Time-Lapse Imaging in Microplates Stem cell imaging requires increased levels of automation and long-term time-lapse capabilities. The FV3000 is designed to image cells over multiple days with accurate timing, low phototoxicity, and accurate focus. Multipoint time-lapse in microplates is routine in stem cell imaging, so the FV3000 can be enhanced with the IX3-ZDC2, Z drift compensator. The IX3-ZDC2 is designed to work with the well navigator, so each well stays in focus during an experiment. For long experiments, add the laser power monitor to maintain consistent laser exposure for excellent laser stability. Users performing stem cell imaging benefit from high-sensitivity detection, silicone objectives, low phototoxicity from the resonant scanner, and the higher throughput from high-speed scanning. Precise stimulation control means photoconversion is simple and efficient, so cells can be reliably stimulated and imaged over multiple days for cell lineage tracking. Whether stem cell cultures are in microplates, single dishes, or microfluidic devices, the FV3000 software and automation makes workflows simple. The stage navigator includes well plate navigation and makes it easy to save, modify, and re-load frequently used plate settings and acquisition conditions. Users can quickly image individual lanes of microfluidic channels. The sequence manager makes it easy to set up longterm time-lapse imaging. Users can adjust the speed and timing of acquisitions while maintaining accurate timing. Quickly visualize and download publication and presentation-ready 3D and 4D image data with the intuitive rendering software included with the FV3000 software suite. Once imaging is completed, the macro functionality in cellsens analysis facilitates 2D cell counting and segmentation with a single mouse click. A spheroid image of a NMuMG cell line expressing Fucci2. Image data courtesy of Atsushi Miyawaki, Cell Function Dynamics, Brain Science Institute of RIKEN. Multipoint time-lapse window IX3-ZDC2 MatTek EpiDermFT Tissue Model: Immunofluorescence labeled with 6 targets of interest. 1. Abcam DRAQ5 ab108410, 2. Abcam Anti- GAPDH (Alexa Fluor 405) ab206372, 3. Abcam Anti- Tubulin (Alexa Fluor 488) ab , 4. Abcam Anti- Fibrillarin (Alexa Fluor 568) ab202540, 5. Abcam Anti- Vimentin (Alexa Fluor 594) ab154207, 6. Abcam Anti- Ki67 (Alexa Fluor 647) ab sample courtesy of MatTek. 9
11 Solutions for Advanced Applications: Spectral Unmixing, Super Resolution, and Photostimulation Both the FV3000 and FV3000RS have a range of standard and optional advanced application features including Olympus Super Resolution (FV-OSR), photostimulation, spectral unmixing, and an external beam combiner. With precise laser control and Olympus patented super resolution method, the FV3000 Series can acquire images with a resolution down to 120 nm, similar to structured illumination methods. Spectral unmixing is robust for a range of applications while photoconversion and photostimulation are efficient and precise, enabling high-speed targeted path scanning and stimulation mapping studies. The Sequence Manager makes it easy to reliably achieve complex cell cycle imaging protocols. Advanced applications, such as random access or targeted path scanning, enable high signal-to-noise multipoint fluorescence measurements for in vitro neuronal cell signaling studies while real-time processing and triggering help provide accurate and coordinated timing control for TTL-driven perfusion devices, stimulators, or other 3rd party peripherals. Macro to micro functionality is easy with the FV3000 Series thanks to the stage navigator, automation built into the IX83 microscope, and the ability to save and reload software layouts, workflows, and experiment conditions. Spectral Umixing Brainbow AAV transfection of Purkinje cells, amplified with antibodies as described in Cai et al Visible are Purkinje cell somata, dendrites and axons, as well as some aspecific stainings of granule cells. Photoconversion and Stimulation Super Resolution Intensity CONVENTIONAL CONFOCAL FV-OSR Time(μs) Trachea multi-ciliated epithelial cells (Culture) Immunofluorescence microscopy: Odf2 staining (Alexa Fluor 488, green) of cilia at the upper part of the basal body (green). Staining for ZO-1 revealed the tight junctions (magenta). Objective: UPLSAPO60XS Image data courtesy of Hatsuho Kanoh, Elisa Herawati, Sachiko Tsukita,Ph. D. Graduate School of Frontier Biosciences and Graduate School of Medicine, Osaka University. 10
12 FV3000 with Galvanometer Scanner to FV3000RS with Resonant Hybrid Scanner: Flexible Configurations to Advance Science High-Speed Resonant Scanning up to 438 Frames per Second Page13 Flexible Detection Lightpath with Wide Dynamic Range Photomultiplier Tubes (PMTs) or High Signal-to-Noise, Cooled GaAsP Spectral Detection Concept (2 4 Channels) Page14 Multichannel Spectral Detector with 16-Channel Unmixing Page14 Combiner System Featuring Diode Lasers with a Range of Wavelengths Page21 Advanced Olympus Optics Page20 Z Drift Compensator IX3-ZDC2 Page21 Precise Ultrasonic Stage IX3-SSU for Multi-Area Imaging Page21 No Darkroom Required Page20 11
13 Powerful, Intuitive Software Page15 Precise Sequence Manager and Real-Time Acquisition Page17 Well Navigator for Microplate, Multipoint Time-Lapse Imaging, and Stitching Page17 Powerful One-Click cellsens Macro Analysis Page17 Olympus Super Resolution with Up to 4 Simultaneous Channels Page19 FV3000RS 12
14 The Right Mixture of Speed and Accuracy The FV3000 Series Scan Units Galvanometer and Galvo/Resonant Hybrid Scanner Users have their choice of two different types of scan units: galvanometer only with the FV3000 or galvanometer/ resonant hybrid with the FV3000RS. The hybrid scan unit has galvanometer scanners for high-precision scanning, as well as a galvo/resonant scanner ideal for high-speed imaging. Galvanometer scanner enables Olympus super resolution technology (FV-OSR) yields resolutions down to 120 nm as well as high signal-to-noise, with precise tornado and multipoint stimulation and 100 ms switching time. Galvanometer scanning can achieve 16 frames per second at 2X zoom. The resonant scanner is capable of speeds ranging from 30 frames per second at to 438 frames per second at Optimized for Live Cell Imaging Resonant scanning greatly reduces photobleaching and phototoxicity compared to standard galvanometer scans by preventing the excitation of fluorophores into triplet states that create reactive oxygen species. These features make live cell experiments more robust and reliable. The FV3000 Series has complete high and low range laser intensity control enabling the system to use the minimum required amount of laser power on samples. The optional Laser Power Monitor provides consistent laser power during long-term time-lapse imaging across multiple days. No Compromise between Speed and Field of View Many high-speed scanning methods restrict the field of view, limiting their usefulness for examining large areas with multiple cells. The FV3000 Series resonant scanner maintains a full 1X field of view, even at a video rate of 30 frames per second. Additional speed is generated by clipping the Y axis, even at 438 frames per second. Platelets bound to thrombosis in blood vessel of mouse. Images taken 30 fps in full frame by resonant scanner with 2 CH GaAsP PMTs. Image data courtesy of Dr. Takuya Hiratsuka, Dr. Michiyuki Matsuda, Graduate School of Biostudies, Kyoto University. Other common scanners FV3000 FN 18, 8 KHz Most resonant scanners force a trade-off between speed and field of view. FLUOVIEW systems are optimized to maintain the field of view with even signal intensity so dynamic samples (e.g. calcium imaging) can be seen in the broad context of their cells and tissues. The image above shows examples of the zoom factors required in other systems. A431 cells fixed with methanol labeled with Abcam Anti-ERK1 + ERK2 antibody (Alexa Fluor 488) ab208564, and Anti-alpha Tubulin antibody (Alexa Fluor 594) ab and DAPI. Sample courtesy of Abcam. 13
15 Introducing TruSpectral Detection A Fully Spectral System with Sensitivity and Accuracy The FV3000 Series employs Olympus TruSpectral detection concept. Based on patented* Volume Phase Hologram (VPH) transmission and an adjustable slit to control light, the spectral detection in FV3000 and FV3000RS is highly efficient, enabling users to select the detection wavelength of each individual channel to 2 nm. * US B/JP B/EP A Tube Lens Adjustable slit Efficient TruSpectral Detection System The FV3000 Series brings new levels of total system transmission efficiency, enabling every system to be completely spectral, improving overall sensitivity, and improving the signal-to-noise ratio for improved multi-color confocal imaging. Efficiency ratio Transmission Efficiency Ratio of FV3000 to FV1200 (Normalization FV1200 as 1.0) FV3000 FV Wavelegnth (nm) VPH High-Sensitivity Spectral Detector (HSD) with GaAsP Photomultiplier Tubes Enhances Quantum Efficiency HSD makes it possible to view samples that were too dim to view with conventional equipment. The GaAsP PMT incorporates 2 channels with a maximum quantum efficiency of 45 %, and Peltier cooling reduces background noise by 20 % for high S/N ratio images under exceptionally low excitation light. Multichannel TruSpectral Detection with 16-Channel Unmixing TruSpectral s efficient design and software enable spectral detectors to run in multichannel mode for both live and postprocessing spectral unmixing with a multichannel lambda mode. Multichannel mode facilitates constant spectral unmixing during live cell experiments, separating complex fluorescence during acquisition. With up to 4 different dynamic ranges from the 4 different channels of array, even bright and dim spectral signals can be separated by adjusting the sensitivity of each detector independently. Standard Quantum Efficiencies of Detector Technologies Quantum Efficiency (%) Multi-Alkali PMT GaAsP PMT Sensitivity Adjustment of Each Channel Spectral Unmixing Wavelength (nm)
16 From Basic to Advanced Acquisition and Analysis, an Interface that Adapts to Your Workflow Intuitive Workflow Customizable and saveable layouts make it easy to tailor the interface to your workflow and experiment needs, from basic to complex. 1. Layout Start by selecting your preferred display with specific tools for basic to complex acquisition. 2. Acquisition Condition Reload settings that were ideal for your last experiment to provide consistency. 3. Acquisition Activate basic to complex acquisitions with live ratio, intensity modulated display, quantitative region of interest (ROI) graphing or spectral unmixing display, and data backup for added security. 15
17 4.Viewer Review data as it is generated. Generate 3D and 4D views and animations to explore and share data in depth. 5. Analysis Extract data from images using online or offline processing. Analytical tools include Olympus super resolution technology (FV-OSR) and powerful cellsens software with features such as deconvolution, filtering, count and measure, and one-click macros. Live Spectral Unmixing with TruSpectral Detection Sequence Manager Page17 Page17 Stage Control for Multipoint Time-Lapse and Microplate Page17 Hard Drive Data Backup Page17 One-Click Macro Analysis Page17 Ratio Imaging and Intensity Modulated Display (IMD) Page18 Rolling Average Processing Page18 Deconvolution Page18 FV-OSR (Olympus Super Resolution) Technology Page19 Macro to Micro Observation Page19 A431 cells fixed with 100 % methanol. Abcam Anti- Integrin alpha 2 antibody (Alexa Fluor 488) ab208770, and Anti-alpha Tubulin antibody (Alexa Fluor 594) ab and DAPI. Sample courtesy of Abcam. 16
18 Intuitive Stage Control, Live Spectral Unmixing, Real-Time Acquisition Live Spectral Unmixing with TruSpectral Detection and Real-Time Processing The power of TruSpectral detection plus multichannel mode means live spectral unmixing can be performed during image acquisition, providing real-time processing of complex overlapping spectra. Stage Control for Multi-Area Time-Lapse, Microplate, and Stitching Microplate imaging is important for many applications, and the Well Navigator provides sophisticated, intuitive controls for a wide range of cell culture vessels and custom plates. Multi-area timelapse and stitching provide robust and accurate time-lapse data. Live blind unmixing of CFP (endosomes, blue), mametrine (plasma membrane, green), mko (nucleus, orange) and mkeima (F-actin, purple) during time-lapse imaging. Image data courtesy of Dr. Kazuhiro Aoki, Dr. Michiyuki Matsuda, Graduate School of Medicine, Kyoto University. Maintain Focus with Z Drift Compensation (ZDC) System The IX3-ZDC2 uses minimally-phototoxic IR light (laser class 1) to identify the location of the sample plane. One-shot autofocus (AF) mode allows several focus positions to be set as desired for deeper samples, enabling efficient Z-stack acquisitions in multi position experiments. Continuous AF mode keeps the desired plane of observation precisely in focus, avoiding focus drift due to temperature changes or the addition of reagents, making it ideal for measurements that requires more stringent focusing. Furthermore, increased optical offset enables continuous AF over plastic vessels or with dry objectives. The IX3-ZDC2 is also compatible with silicone objectives (in AF mode). IX3-ZDC2 Optical Path Diagram cell Hard Disk Recording The microscope comes equipped with a hard-disk drive (HDD) recording function. The images captured are stored automatically in the HDD. Large volumes of data, such as those obtained from long-term time-lapse imaging can be stored. Powerful One-Click Macro Analysis with cellsens Images alone are not enough; with integrated cellsens Count and Measure analysis, the FV3000 Series can optimize images with deconvolution and analyze them with one-click macro functionality for a broad range of morphological measurements. Offset AF sensor Offset lens Cover glass Objective Precise Sequence Manager and Real-Time Acquisition Complex protocols are handled with ease, and real-time control helps provide microsecond accuracy of scans with millisecond accuracy over days of time-lapse. A spheroid image of a NMuMG cell line expressing Fucci2. Image data courtesy of Atsushi Miyawaki, Cell Function Dynamics, Brain Science Institute of RIKEN. 17
19 Additional Intuitive Features Ratio Imaging and Intensity Modulated Display (IMD) The FV3000RS includes an Intensity Modulated Display (IMD) function in the software that displays quantitative fluorescence ratio changes during both standard and high-speed acquisitions. This function is particularly useful for calcium and FRET imaging where a pure ratio display provides poor contrast in background areas. Rolling Average Processing High-speed scanning at low laser power to avoid phototoxicity often decreases the signal-to-noise ratio. With rolling average post-processing, users have the flexibility to adjust high-speed time-lapse images while maintaining time scale and keeping the original data. Ratio(ex405/ex488) High Raw 30 fps data acquired at low laser power (0.05 %, 488 nm). Rolling average processing (10 frame) on 30 fps data acquired at low laser power. Low Ratio(ex405/ex488) 10 μm CCCP treatment Deconvolution The optional Constrained Iterative (CI) Deconvolution Solution employs advanced CI algorithms to produce improved resolution, contrast, and dynamic range, with industry-leading speed. This proprietary post-processing tool is efficient for both CCD and confocal imaging and enhances the ability to differentiate between imaged objects. tsgfp1-mito reveals heterogeneity in mitochondrial thermogenesis in HeLa cells. The images of ratio (ex 405 nm/ex 488 nm) in tsgfp1-mito-expressing cells before and after CCCP treatment at 37 C. Scale bars indicate 10 μm (whole image) and 3 μm (inset). Image data courtesy of Shigeki Kiyonaka Ph,D, Yasuo Mori Ph,D Molecular Biology Field, Department of Synthetic Chemistry and Biological Chemistry, Kyoto University. Original Image Deconvolved Image CFP YFP FRET Cell line: Human cervical cancer cell line HeLa cell Immunostaining: Hec1 staining (green, Alexa Fluor 488), α-tubulin staining (red, Alexa Fluor 568),DAPI staining (blue) Mitotic HeLa cell derived from human cervical cancer. Mitotic spindle and kinetochores are stained with anti-α-tubulin (red) and anti-hec1 (green) antibodies, respectively. Chromosomes interact with microtubules constituting mitotic spindle via kinetochores, protein structure assembled on centromere region of chromosomes. Image data courtesy of Masanori Ikeda and Kozo Tanaka, Department of Molecular Oncology, Institute of Development, Aging and Cancer, Tohoku University. Raw CFP/YFP ratio IMD of CFP/YFP ratio Cardiomyoctye Image data courtesy of Yusuke Niino and Atsushi Miyawaki, Cell Function Dynamics, Brain Science Institute of RIKEN. 18
20 Olympus Super Resolution Technology Olympus Super Resolution (FV-OSR) Technology with Up to 4 Simultaneous Channels Olympus widely applicable super resolution method requires no special fluorophores and works for a wide range of samples. Ideal for colocalization analysis, the FV-OSR can acquire 4 fluorescent signals either sequentially or simultaneously with a resolution of approximately 120 nm*, nearly doubling the resolution of typical confocal microscopy. The system is easy to use with minimal user training and can be added to any confocal system, making the FV-OSR a truly accessible method for achieving super resolution. * Subject to objective magnification, numerical aperture, excitation and emission wavelength, and experiment conditions. Beyond Deconvolving Confocal: Comparison of Confocal, Deconvolved Confocal and Deconvolved FV-OSR Images 0.5 AU Confocal Image Enlargement 0.5 AU Confocal Image Deconvolved with cellsens Advanced Deconvolution Olympus Super Resolution Plus cellsens Advanced Deconvolution Secondary antibody labels against GFP (Alexa Fluor 488, neurons) and SV2 (Alexa Fluor 565, red). Sample courtesy of Dr. Ed Boyden and Dr. Fei Chen, MIT. Macro to Micro Observation Finding areas of interest in samples can be challenging. The confocal optical design of the FV3000 Series supports macro to micro imaging so users can quickly switch from low magnification overview observation with 1.25X objectives to high-magnification, detailed observation with up to 150X objectives. Users can employ image stitching at both macro and micro levels to generate overview images that show samples in context. A stitched image of a coronal section (30 μm thickness) from an adult YFP-H mouse cerebrum acquired with 20X objective (UPLSAPO20X). Image data courtesy of Takako Kogure and Atsushi Miyawaki, Cell Function Dynamics, Brain Science Institute of RIKEN. 19
21 Superior Optics and a Rigid Frame Ideal for Live Cell Imaging Silicone Immersion Objectives for Live Cell Imaging Deliver High-Resolution Observation at Depth Olympus offers four high NA silicone immersion objectives that deliver excellent performance for live cell imaging. The refractive index of silicone oil (ne 1.40) is close to that of living tissue (ne 1.38), enabling high-resolution observations deep inside living tissue with minimal spherical aberration caused by refractive index mismatch. Silicone oil does not dry out or harden, so there is never a need to refill oil, making it ideal for extended time-lapse observations. UPLSAPO30XS: For a broader view and greater depth Magnification: 30X, NA: 1.05 (silicone oil immersion), W.D.: 0.8 mm, cover glass thickness: mm, operating temperature: C Refractive Index is Important with Deep Tissue Observation In deep tissue observation, image quality depends on keeping the refractive index of the sample and immersion medium as close to each other as possible. UPLSAPO40XS : Complete the magnification range Magnification: 40X, NA: 1.25 (silicone oil immersion), W.D.: 0.3 mm, cover glass thickness: mm, operating temperature: C UPLSAPO60XS2: For 3D observations with superior resolution Magnification: 60X, NA: 1.30 (silicone oil immersion), W.D.: 0.3 mm, cover glass thickness: mm, operating temperature: C Water ne 1.33 Sample ne 1.38 Cover glass ne 1.52 Silicone oil ne 1.40 UPLSAPO100XS: For greater depth in closely defined regions Magnification: 100X, NA: 1.35 (silicone oil immersion), W.D.: 0.2 mm, cover glass thickness: mm, operating temperature: C Water immersion objective Silicone immersion objective When working with a water immersion When working with a silicone immersion objective, the difference between the objective, the difference between the refractive index of the samples and water refractive index of the samples and silicone results in spherical aberration in deep oil is minimal. So it achieves brighter tissue, causing resolution to deteriorate and fluorescence images with higher resolution fluorescence to become dim. for deep tissue. PLAPON60XOSC2: Enhance the Reliability of Colocalization Analysis with a Low Chromatic Aberration Objective This oil immersion objective minimizes lateral and axial chromatic aberration in the nm spectrum. Colocalization images are acquired reliably and images are measured with superior positional accuracy. The objective also compensates for chromatic aberration through near infrared up to 850 nm, making it the ideal choice for quantitative imaging. Performance Comparison of PLAPON60XOSC2 and UPLSAPO60XO PLAPON UPLSAPO 60XOSC2 60XO Axial chromatic aberration (Z direction) Compared for PSF fluorescent beads (405 nm, 633 nm) Approx. 0 μm Approx. 0.5 μm Low Chromatic Aberration Objective Magnification: 60X NA: 1.4 (oil immersion) W.D.: 0.12 mm Chromatic aberration compensation range: nm Optical data provided for each objective. Lateral chromatic aberration (X-Y direction) Compared for PSF fluorescent beads (405 nm, 488 nm, 633 nm) 3D image Tubulin in Ptk2 cells labeled with two colors (405 nm, 635 nm) and compared Approx. 0.1 μm Approx. 0.2 μm Meeting the Requirements of Stability with the IX83 A Z-drive guide installed near the revolving nosepiece combines high thermal rigidity with the stability of a wraparound structure to significantly reduce the impact of heat and vibration and improve the quality of time-lapse imaging. High Contrast under Bright Conditions The umbra unit is designed specifically for fluorescence observation. It efficiently blocks out room light, enhances the contrast of fluorescence, and enables clear fluorescence observation under bright conditions. Thermal Drift Displacement Periodic Damping IX81 IX81 Square Frame for Increased Rigidity IX83 IX (min) (s) 20
22 Modular Units Designed for Your Applications Scanners Laser Combiners Other Equipment Choose from the following options with fieldupgradable laser-based autofocus, fast and precise motorized stage control, analog input/output and TTL synchronization, and a convenient anti-vibration platform. Hybrid Scan Unit (Resonant/Galvanometer) The hybrid scanner combines the capabilities of a galvanometer scanner with a resonant scanner for high-speed imaging in the full field of view at 30 fps and up to 438 fps at The Sequence Manager makes it simple to automatically switch between resonant and galvanometer imaging in the same experiment. Galvo Scan Unit The galvanometer-only scanner provides precision scanning from 1 fps at to 16 fps. High-speed multipoint stimulation or detection experiments can travel between multiple cells at over 100 Hz with data output as high as 500 khz. Spectral Detectors Main Laser Combiner The main laser combiner is the heart of the laser system. Four standard lasers with an option to add a fifth laser or leave an open port to add an additional three diode lasers via the Sub Combiner. Sub Laser Combiner Add this optional combiner at any time with up to 3 diode lasers for a maximum of 7 laser lines in combination with the main laser combiner. Illumination Units The conventional illumination modules are designed for long-duration time-lapse experiments. Since light is introduced through fiber delivery systems, no heat is transferred to the microscope. Z Drift Compensator/IX3-ZDC2 The IX3-ZDC2 uses minimally-phototoxic IR light to identify the location of the sample plane. The IX3-ZDC2 is also compatible with silicone objectives and plastic bottom vessels. Ultrasonic Stage for IX3/IX3-SSU With low thermal drift for improved accuracy, the ultrasonic stage can be controlled by both software and Touch Panel Control for fast, reliable multi-area imaging. High Sensitivity Spectral Detector (GaAsP PMT) with TruSpectral Technology The 2-channel High Sensitivity Spectral Detector (HSD) employs the same Volume Phase Holographic (VPH) technology as the SD, with Peltier cooled GaAsP PMTs and a high quantum efficiency of 45 % and detection up to 750 nm. This unit can be combined with the 2-channel spectral detector (SD) for a flexible dynamic range or a second 2-channel HSD unit for powerful 4-channel sensitivity. Spectral Detector (Multialkali PMT) with TruSpectral Technology The 2-channel SD employs efficient VPH transmission and an adjustable slit with nm bandwidth from nm detection. The multialkalai PMTs provide a broad dynamic range for detection up to 800 nm. Light Source/U-HGLGPS The pre-centered fluorescence illumination source requires no adjustment and has an average lifespan of 2,000 hours. Transmitted Detector/FV31-LETD This unit combines an external transmitted light photomultiplier detector and LED conventional illumination for both laser scanning and conventional transmitted light Nomarski DIC observation. Users can undertake simultaneous multichannel confocal fluorescence imaging and transmitted DIC acquisition. IO Interface Box/FV30-ANALOG This unit supports electro-physiological experiments through analog inputs and TTL I/ O support. The interface box converts voltage to images that can be treated in the same manner as fluorescence images. Simple Anti-Vibration Plate/FV31-AVP Designed to match the footprint of the FV3000, this simple anti-vibration plate provides a compact solution for those who do not need a full anti-vibration table. 21
23 Specifications FLUOVIEW FV3000 Specifications FV3000 FV3000RS Laser Light Violet/Visible Light Laser 405 nm: 50 mw, 488 nm: 20 mw, 561 nm: 20 mw, 640 nm: 40 mw One optional laser port for sub laser combiner or optional laser unit Optional Laser Sub Laser Combiner Laser as follows (max. 3 laser units ) 445 nm: 75 mw, 514 nm: 40 mw, 594 nm: 20 mw, connected to main laser combiner Single Laser Unit 445 nm: 75 mw, 514 nm: 40 mw, or 594 nm: 20 mw, directly connected to main laser combiner Laser Light Control Main laser combiner with implemented AOTF system, ultra-fast intensity modulation with individual laser lines, additional shutter control continuously variable (0.1 % 100 %, 0.1 % increments) 10 % or 100 % maximum laser power changer by ND filter Scanner Scanning Method 2 silver-coated galvanometer scanning mirrors 2 silver-coated galvanometer scanning mirrors 1 silver-coated resonant and 1 silver-coated galvanometer scanning mirrors. High Sensitivity- Spectral Detector Spectral Detector Galvanometer Scanner (Normal Imaging) Resonant Scanner (High-Speed Imaging) Pinhole Scanning Resolution: to pixels Scanning Speed (One Way): with 1.1 s 264 s. pixel time : 2 μs 1000 μs. Scanning Speed (Round Trip): with 63 ms ms with 16 ms ms Optical Zoom: 1X 50X in 0.01X increments Scan Rotation: Free rotation (360 degree) in steps of 0.1 degree Scanning Mode: PT, XT, XZ, XY, XZT, XYT, XYZ, XYλ, XYZT, XYλT, XYλZ, XYλZT ROI Scanning, rectangle clip, ellipse, polygon, free area, line, free line and point, tornado mode only for stimulation Scanning Resolution: to pixels Scanning Speed: 30 fps at , 438 fps at Optical Zoom: 1X 8X in 0.01X increments Scanning Mode: XT, XZ, XY, XZT, XYT, XYZ, XYλ, XYZT, XYλT, XYZ, XYλZT ROI Scanning, Rectangle Clip, Line Single motorized pinhole, pinhole diameter ø μm (1 μm Steps) Field Number (FN) 18 Dichromatic Mirror Turret 8 positions (high performance DMs and 10/90 mirror) Optional Unito for Scanner Laser Power monitor, optional laser port Detector Module Cooled GaAsP photomultiplier, 2 channels Spectral Method Motorized Volume Phase Holographic transmission diffraction grating, motorized adjustable slit, selectable wavelength bandwidth: nm, wavelength resolution: 2 nm Dichromatic Mirror Turret 8 positions (high performance DMs and mirror) Detector Module Multi-Alkali photomultiplier, 2 channels Spectral Method Motorized Volume Phase Holographic transmission diffraction grating, motorized adjustable slit selectable wavelength bandwidth: nm, wavelength resolution: 2 nm Dichromatic Mirror Turret 8 positions (high performance DMs and mirror) Microscope Motorized Microscope Inverted IX83 (IX83P2ZF) Integrated motorized focus module, minimum increment 0.01 μm System Control Control Unit OS: Windows 7 Professional 64-bit (English version), built-in dedicated I/F board and hardware sequencer for precise imaging timing Display 30 or 32-inch monitor (WQUXGA ) Fluorescence Illumination Unit External fluorescence light source, fiber adapter to optical port of scan unit, motorized switching between LSM light path and fluorescence illumination Transmitted Light Detector Unit Module with integrated external transmitted light photomultiplier detector and LED lamp, motorized switching Software Basic Features 2D Image Display 3D Visualization and Observation Image Format Spectral Unmixing Image Analysis Statistical Processing Optional Software GUI designed for darkroom environment. User-arrangeable layout. Acquisition parameter reload features. Hard disk recording capability, adjust laser power and HV with Z-stack acquisition. Z-stack with alpha blending, maximum-intensity projection, iso-surface rendering. Each image display: single-channel side-by-side, merge, cropping, live tiling, live tile, series (Z/T/λ), LUT: individual color setting, pseudo-color, comment: graphic and text input Interactive volume rendering: volume rendering display, projection display, animation displayed. 3D animation (maximum intensity projection method, α blending) 3D and 2D sequential operation function OIR image format 8/16-bit gray scale/index color, 24/ 32/ 48-bit color, JPEG/ BMP/ TIFF image functions, Olympus multi-tif format Fluorescence spectral unmixing modes (up to 16 channels) Fluorescence intensity and time-lapse measurement 2D data histogram display Motorized-stage control Mapping and multiplepoint simulation Sequence manager Virtual channel acquisition Microplate navigation Remote development kit Super resolution imaging (FV-OSR) World Wide Support Installation generally takes one day to get systems up and running fast. We support our products via our global knowledge base. Olympus application specialists can assist you with choosing the features that will optimize your system for your applications. Confocal systems are an investment, and keeping the system running in the best performance is important. Our certified service teams can deploy rapid alignment procedures and system diagnostics to keep your system in top shape and diagnose any issues. 22
24 Image data are courtesy of the following institutions: Mouse kidney (cover and page 2) and rat embryo sample (3, page 1) prepared by Dr. Mike Davidson. Images presented with lasting gratitude for his lifetime commitment to science and microscopy. Whole mouse kidney captured in single shot with 1.25X objective. 10 μm section, TOMM20 ATTO 647N, Phalloidin Alexa Fluor 568, WGA Alexa Fluor 488, DAPI. (cover page and page 2) 3D rendered image of Xenopus endoderm labeled with malachite green and methylene blue. 3 channel image captures label and autofluorescence. (1, page 1) 3D rendered image of Xenopus endoderm labeled with malachite green and methylene blue. (2, page 1) 2 2 tiled image of whole rat embryo, 20 mm total field of view. H&E fluorescence with 640 nm laser diode. (3, page 1) Growing HeLa cells expresses Fucci, a cell cycle indicator. Fluorescense image (1, page 3), Cell counting (2,3, page 3) Asako Sakae-Sawano, Atsushi Miyawaki, Cell Function Dynamics, Brain Science Institute of RIKEN. 3D Time-lapse of mouse embryonic fibroblast labeled with silicone rhodamine docetaxol (Tubulin, white), RFP centrin (green) imaged with 100X silicone objective and 30 fps resonant scanning followed by cellsens deconvolution. Dr. Markus Delling, Harvard University. (4, page3) ScaleA2-treated neocortex Motokazu Uchigashima, M.D., Ph.D., Masahiko Watanabe, M.D., Ph.D., Departments of Anatomy, Hokkaido University Graduate School of Medicine. (5,6, page 3) A stitched image of a coronal section (30 μm thickness) from an adult YFP-H mouse cerebrum acquired with 20X objective (UPLSAPO20X). Takako Kogure and Atsushi Miyawaki, Cell Function Dynamics, Brain Science Institute of RIKEN. (7, page3) Platelets bound to thrombosis in blood vessel of mouse. Images taken 30 fps in full frame by resonant scanner with 2 CH GaAsP PMTs. Dr.Takuya Hiratsuka, Dr. Michiyuki Matsuda, Graduate School of Biostudies, Kyoto University. (8, page 3) FRET spectral look up table display of cardiac myocyte (9, page 3), CFP spectral look up table display of cardiac myocyte (10, page 3), Differential Interference Contrast (DIC) image of cardiac myocyte (11, page 3), Overlay (12, page 3), IMD ratio images of spontaneous Ca 2+ oscillation in a beating rat cardiomyocyte expressing yellow cameleon. (13, page 3) Yusuke Niino and Atsushi Miyawaki, Cell Function Dynamics, Brain Science Institute of RIKEN. Fucci induced Spheroid of HT29 cell line Yuji Mishima, Ph.D., Kiyohiko Hatake M.D., Ph.D. Clinical Chemotherapy, Cancer Chemotherapy Center, Japanese Foundation for Cancer Research. (14, page 4) A spheroid image of a NMuMG cell line expressing Fucci2. Atsushi Miyawaki, Cell Function Dynamics, Brain Science Institute of RIKEN. (15, page 4) FRET imaging by expressed Raichu-Cdc42 in cultured HT1080. Activated Cdc42 is observed to the cell moving direction. Ms. Satsuki Fujiwara and Dr. Michiyuki Matsuda, Graduate school of Biostudies, Kyoto University. (16, page 4) Brainbow AAV transfection of Purkinje cells, amplified with antibodies as described in Cai et al Visible are Purkinje cell somata, dendrites and axons, as well as some aspecific staining of granule cells. (17, page 4) Cultured epithelial HeLa (EpH) cells. Immunofluorescence microscopy: α-tubulin staining (Alexa Fluor 488, green), ZO-1 staining (Alexa Fluor 568, magenta) Staining for ZO-1 revealed the tight junctions (TJs) (magenta). Staining for α-tubulin showed an apical network of microtubules. This network associates with the TJ to form the TJ-apical complex (green). Objective: UPLSAPO100XS Image data Courtesy of Hatsuho Kanoh, Tomoki Yano, Sachiko Tsukita,Ph.D. Graduate School of Frontier Biosciences and Graduate School of Medicine, Osaka University. (18, page 4) is ISO14001 certified. is ISO9001 certified. Illumination devices for microscope have suggested lifetimes. Periodic inspections are required. Please visit our website for details. All company and product names are registered trademarks and/or trademarks of their respective owners. Images on the PC monitors are simulated. Specifications and appearances are subject to change without any notice or obligation on the part of the manufacturer. This product is designed for use in industrial environments for the EMC performance. Using it in a residential environment may affect other equipment in the environment. Shinjuku Monolith, Nishi-Shinjuku, Shinjuku-ku, Tokyo , Japan 5301 Blue Lagoon Drive, Suite 290 Miami, FL 33126, U.S.A. For enquiries - contact Wendenstrasse 14-18, Hamburg, Germany 48 Woerd Avenue, Waltham, MA 02453, U.S.A. 491B River Valley Road, #12-01/04 Valley Point Office Tower, Singapore B, First Floor, Time Tower, M.G. Road, Gurgaon , Haryana, INDIA A8F, Ping An International Financial Center, No. 1-3, Xinyuan South Road, Chaoyang District, Beijing, P.R.C. 8F Olympus Tower, 446 Bongeunsa-ro, Gangnam-gu, Seoul, Korea 3 Acacia Place, Notting Hill VIC 3168, Australia Printed in Japan N
Confocal Laser Scanning Microscope FV3000 FLUOVIEW. Next Generation FLUOVIEW for the Next Revolutions in Science
 Confocal Laser Scanning Microscope FV3000 FLUOVIEW Next Generation FLUOVIEW for the Next Revolutions in Science (1) (2) (3) 1 FLUOVIEW FV3000 Laser Scanning Microscope The FLUOVIEW FV3000 Series is designed
Confocal Laser Scanning Microscope FV3000 FLUOVIEW Next Generation FLUOVIEW for the Next Revolutions in Science (1) (2) (3) 1 FLUOVIEW FV3000 Laser Scanning Microscope The FLUOVIEW FV3000 Series is designed
Quality Performance, Innovative Design
 Dimensions Confocal Laser Scanning Biological Microscope Table size (mm): 1400(W) 800(D) * Table is not available from Olympus. Avoid placing the controller directly on the floor. Dimensions / Weight /
Dimensions Confocal Laser Scanning Biological Microscope Table size (mm): 1400(W) 800(D) * Table is not available from Olympus. Avoid placing the controller directly on the floor. Dimensions / Weight /
 07 Setting Place a specimen, and select a fluorescence dye. The FV10i automatically selects the most suitable imaging conditions based on the fluorescence dye selection. Set Image mapping menu Just click
07 Setting Place a specimen, and select a fluorescence dye. The FV10i automatically selects the most suitable imaging conditions based on the fluorescence dye selection. Set Image mapping menu Just click
Opterra II Multipoint Scanning Confocal Microscope. Innovation with Integrity
 Opterra II Multipoint Scanning Confocal Microscope Enabling 4D Live-Cell Fluorescence Imaging through Speed, Sensitivity, Viability and Simplicity Innovation with Integrity Fluorescence Microscopy The
Opterra II Multipoint Scanning Confocal Microscope Enabling 4D Live-Cell Fluorescence Imaging through Speed, Sensitivity, Viability and Simplicity Innovation with Integrity Fluorescence Microscopy The
Multifluorescence The Crosstalk Problem and Its Solution
 Multifluorescence The Crosstalk Problem and Its Solution If a specimen is labeled with more than one fluorochrome, each image channel should only show the emission signal of one of them. If, in a specimen
Multifluorescence The Crosstalk Problem and Its Solution If a specimen is labeled with more than one fluorochrome, each image channel should only show the emission signal of one of them. If, in a specimen
Opterra. Multipoint Scanning Confocal Microscope. Innovation with Integrity. Cell-Friendly, High-Speed, High-Resolution Imaging
 Opterra Multipoint Scanning Confocal Microscope Cell-Friendly, High-Speed, High-Resolution Imaging Innovation with Integrity Fluorescence Microscopy Opterra Multipoint Scanning Confocal Microscope Superior
Opterra Multipoint Scanning Confocal Microscope Cell-Friendly, High-Speed, High-Resolution Imaging Innovation with Integrity Fluorescence Microscopy Opterra Multipoint Scanning Confocal Microscope Superior
Why and How? Daniel Gitler Dept. of Physiology Ben-Gurion University of the Negev. Microscopy course, Michmoret Dec 2005
 Why and How? Daniel Gitler Dept. of Physiology Ben-Gurion University of the Negev Why use confocal microscopy? Principles of the laser scanning confocal microscope. Image resolution. Manipulating the
Why and How? Daniel Gitler Dept. of Physiology Ben-Gurion University of the Negev Why use confocal microscopy? Principles of the laser scanning confocal microscope. Image resolution. Manipulating the
Leica TCS SP8 Quick Start Guide
 Leica TCS SP8 Quick Start Guide Leica TCS SP8 System Overview Start-Up Procedure 1. Turn on the CTR Control Box, Fluorescent Light for the microscope stand. 2. Turn on the Scanner Power (1) on the front
Leica TCS SP8 Quick Start Guide Leica TCS SP8 System Overview Start-Up Procedure 1. Turn on the CTR Control Box, Fluorescent Light for the microscope stand. 2. Turn on the Scanner Power (1) on the front
Nature Methods: doi: /nmeth Supplementary Figure 1. Schematic of 2P-ISIM AO optical setup.
 Supplementary Figure 1 Schematic of 2P-ISIM AO optical setup. Excitation from a femtosecond laser is passed through intensity control and shuttering optics (1/2 λ wave plate, polarizing beam splitting
Supplementary Figure 1 Schematic of 2P-ISIM AO optical setup. Excitation from a femtosecond laser is passed through intensity control and shuttering optics (1/2 λ wave plate, polarizing beam splitting
Practical work no. 3: Confocal Live Cell Microscopy
 Practical work no. 3: Confocal Live Cell Microscopy Course Instructor: Mikko Liljeström (MIU) 1 Background Confocal microscopy: The main idea behind confocality is that it suppresses the signal outside
Practical work no. 3: Confocal Live Cell Microscopy Course Instructor: Mikko Liljeström (MIU) 1 Background Confocal microscopy: The main idea behind confocality is that it suppresses the signal outside
Supplemental Figure 1: Histogram of 63x Objective Lens z axis Calculated Resolutions. Results from the MetroloJ z axis fits for 5 beads from each
 Supplemental Figure 1: Histogram of 63x Objective Lens z axis Calculated Resolutions. Results from the MetroloJ z axis fits for 5 beads from each lens with a 1 Airy unit pinhole setting. Many water lenses
Supplemental Figure 1: Histogram of 63x Objective Lens z axis Calculated Resolutions. Results from the MetroloJ z axis fits for 5 beads from each lens with a 1 Airy unit pinhole setting. Many water lenses
Things to check before start-up.
 Byeong Cha Page 1 11/24/2009 Manual for Leica SP2 Confocal Microscope Enter you name, the date, the time, and the account number in the user log book. Things to check before start-up. Make sure that your
Byeong Cha Page 1 11/24/2009 Manual for Leica SP2 Confocal Microscope Enter you name, the date, the time, and the account number in the user log book. Things to check before start-up. Make sure that your
Training Guide for Carl Zeiss LSM 5 LIVE Confocal Microscope
 Training Guide for Carl Zeiss LSM 5 LIVE Confocal Microscope AIM 4.2 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Verify that main power switches on the
Training Guide for Carl Zeiss LSM 5 LIVE Confocal Microscope AIM 4.2 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Verify that main power switches on the
Training Guide for Leica SP8 Confocal/Multiphoton Microscope
 Training Guide for Leica SP8 Confocal/Multiphoton Microscope LAS AF v3.3 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Turn ON power switch for epifluorescence
Training Guide for Leica SP8 Confocal/Multiphoton Microscope LAS AF v3.3 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Turn ON power switch for epifluorescence
Training Guide for Carl Zeiss LSM 510 META Confocal Microscope
 Training Guide for Carl Zeiss LSM 510 META Confocal Microscope AIM 4.2 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Turn ON Components and System/PC switches
Training Guide for Carl Zeiss LSM 510 META Confocal Microscope AIM 4.2 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2017) Power ON Routine 1 2 Turn ON Components and System/PC switches
Spectral Imaging with the Opterra Multipoint Scanning Confocal
 Spectral Imaging with the Opterra Multipoint Scanning Confocal Outline Opterra design overview Scan Modes Light Path Spectral Imaging with Opterra Drosophila larva heart. Opterra Design Overview Supravideo
Spectral Imaging with the Opterra Multipoint Scanning Confocal Outline Opterra design overview Scan Modes Light Path Spectral Imaging with Opterra Drosophila larva heart. Opterra Design Overview Supravideo
LSM 510 META in Chang Gung University
 Content LSM 510 META in Chang ung University LSM 510 META 路 理 The features and applications of LSM 510 META 01-09 Introduction of the hardware 10-12 Fluorescence observation in conventional microscope
Content LSM 510 META in Chang ung University LSM 510 META 路 理 The features and applications of LSM 510 META 01-09 Introduction of the hardware 10-12 Fluorescence observation in conventional microscope
Zeiss 880 Training Notes Zen 2.3
 Zeiss 880 Training Notes Zen 2.3 1 Turn on the HXP 120V Lamp 2 Turn on Main Power Switch Turn on the Systems PC Switch Turn on the Components Switch. 3 4 5 Turn on the PC and log into your account. Start
Zeiss 880 Training Notes Zen 2.3 1 Turn on the HXP 120V Lamp 2 Turn on Main Power Switch Turn on the Systems PC Switch Turn on the Components Switch. 3 4 5 Turn on the PC and log into your account. Start
Leica TCS SP8 Quick Start Guide
 Leica TCS SP8 Quick Start Guide Leica TCS SP8 System Overview Start-Up Procedure 1. Turn on the CTR Control Box, EL6000 fluorescent light source for the microscope stand. 2. Turn on the Scanner Power
Leica TCS SP8 Quick Start Guide Leica TCS SP8 System Overview Start-Up Procedure 1. Turn on the CTR Control Box, EL6000 fluorescent light source for the microscope stand. 2. Turn on the Scanner Power
LSM 710 Confocal Microscope Standard Operation Protocol
 LSM 710 Confocal Microscope Standard Operation Protocol Basic Operation Turning on the system 1. Switch on Main power switch 2. Switch on System / PC power button 3. Switch on Components power button 4.
LSM 710 Confocal Microscope Standard Operation Protocol Basic Operation Turning on the system 1. Switch on Main power switch 2. Switch on System / PC power button 3. Switch on Components power button 4.
Confocal Microscope. Confocal Microscope C2
 Confocal Microscope Confocal Microscope C2 Confocal Microscope An essential microscopy laboratory instrument The C2 confocal microscope system comprises a new generation of Nikon confocal instruments designed
Confocal Microscope Confocal Microscope C2 Confocal Microscope An essential microscopy laboratory instrument The C2 confocal microscope system comprises a new generation of Nikon confocal instruments designed
ImageXpress Micro XLS Widefield High Content Screening System. Imaging with a vision.
 ImageXpress Micro XLS Widefield High Content Screening System Imaging with a vision www.moleculardevices.com The ImageXpress Micro Widefield High Content Screening System is the ultimate combination of
ImageXpress Micro XLS Widefield High Content Screening System Imaging with a vision www.moleculardevices.com The ImageXpress Micro Widefield High Content Screening System is the ultimate combination of
Zeiss 780 Training Notes
 Zeiss 780 Training Notes Turn on Main Switch, System PC and Components Switches 780 Start up sequence Do you need the argon laser (458, 488, 514 nm lines)? Yes Turn on the laser s main power switch and
Zeiss 780 Training Notes Turn on Main Switch, System PC and Components Switches 780 Start up sequence Do you need the argon laser (458, 488, 514 nm lines)? Yes Turn on the laser s main power switch and
The Next Level of TIRF Microscopy. cell^tirf Illuminator Motorized Total Internal Reflection Fluorescence
 cell^tirf Illuminator Motorized Total Internal Reflection Fluorescence Four individually aligned illumination beams for simultaneous multi-color TIRF imaging The Next Level of TIRF Microscopy Mario Faretta,
cell^tirf Illuminator Motorized Total Internal Reflection Fluorescence Four individually aligned illumination beams for simultaneous multi-color TIRF imaging The Next Level of TIRF Microscopy Mario Faretta,
Microscopy from Carl Zeiss
 Microscopy from Carl Zeiss Contents Page Contents... 1 Introduction... 1 Starting the System... 2 Introduction to ZEN Efficient Navigation... 5 Setting up the microscope... 10 Configuring the beam path
Microscopy from Carl Zeiss Contents Page Contents... 1 Introduction... 1 Starting the System... 2 Introduction to ZEN Efficient Navigation... 5 Setting up the microscope... 10 Configuring the beam path
contents TABLE OF The SECOM platform Applications - sections Applications - whole cells Features Integrated workflow Automated overlay
 S E C O M TABLE OF contents The SECOM platform 4 Applications - sections 5 Applications - whole cells 8 Features 9 Integrated workflow 12 Automated overlay ODEMIS - integrated software Specifications 13
S E C O M TABLE OF contents The SECOM platform 4 Applications - sections 5 Applications - whole cells 8 Features 9 Integrated workflow 12 Automated overlay ODEMIS - integrated software Specifications 13
START-UP PROCEDURE 1 THE MICROSCOPE STAND 3 OBJECTIVES 5 STARTING WITH LAS (SOFTWARE) AND SETTING UP THE MICROSCOPE STAND 7
 Leica DMI AF6000LX Table of contents START-UP PROCEDURE 1 THE MICROSCOPE STAND 3 OBJECTIVES 5 STARTING WITH LAS (SOFTWARE) AND SETTING UP THE MICROSCOPE STAND 7 ACQUIRE MODULE 6 SETTING THE LIGHTPATH 6
Leica DMI AF6000LX Table of contents START-UP PROCEDURE 1 THE MICROSCOPE STAND 3 OBJECTIVES 5 STARTING WITH LAS (SOFTWARE) AND SETTING UP THE MICROSCOPE STAND 7 ACQUIRE MODULE 6 SETTING THE LIGHTPATH 6
ZEISS LSM510META confocal manual
 ZEISS LSM510META confocal manual Switching on the system 1) Switch on the Remote Control button located on the table to the right of the microscope. This is the main switch for the whole system including
ZEISS LSM510META confocal manual Switching on the system 1) Switch on the Remote Control button located on the table to the right of the microscope. This is the main switch for the whole system including
LSM 780 Confocal Microscope Standard Operation Protocol
 LSM 780 Confocal Microscope Standard Operation Protocol Basic Operation Turning on the system 1. Sign on log sheet according to Actual start time 2. Check Compressed Air supply for the air table 3. Switch
LSM 780 Confocal Microscope Standard Operation Protocol Basic Operation Turning on the system 1. Sign on log sheet according to Actual start time 2. Check Compressed Air supply for the air table 3. Switch
Boulevard du Temple Daguerrotype (Paris,1838) a busy street? Nyquist sampling for movement
 Boulevard du Temple Daguerrotype (Paris,1838) a busy street? Nyquist sampling for movement CONFOCAL MICROSCOPY BioVis Uppsala, 2017 Jeremy Adler Matyas Molnar Dirk Pacholsky Widefield & Confocal Microscopy
Boulevard du Temple Daguerrotype (Paris,1838) a busy street? Nyquist sampling for movement CONFOCAL MICROSCOPY BioVis Uppsala, 2017 Jeremy Adler Matyas Molnar Dirk Pacholsky Widefield & Confocal Microscopy
Nikon AZ100. Laser Scanning Macro Confocal Microscope. Jordan Briscoe Adam Fries Kyle Marchuk Kaitlin Corbin. May 2017.
 Nikon AZ100 Laser Scanning Macro Confocal Microscope Jordan Briscoe Adam Fries Kyle Marchuk Kaitlin Corbin May 2017 Contents 1 Introduction 2 2 Hardware - Startup 2 3 Software/Operation 4 3.1 Multidimensional
Nikon AZ100 Laser Scanning Macro Confocal Microscope Jordan Briscoe Adam Fries Kyle Marchuk Kaitlin Corbin May 2017 Contents 1 Introduction 2 2 Hardware - Startup 2 3 Software/Operation 4 3.1 Multidimensional
Working Simultaneously. The Next Level of TIRF Microscopy. cell^tirf Illuminator Motorized Total Internal Reflection Fluorescence
 cell^tirf Illuminator Motorized Total Internal Reflection Fluorescence Four individually aligned illumination beams for simultaneous multi-color TIRF imaging Working Simultaneously The Next Level of TIRF
cell^tirf Illuminator Motorized Total Internal Reflection Fluorescence Four individually aligned illumination beams for simultaneous multi-color TIRF imaging Working Simultaneously The Next Level of TIRF
Confocal Microscope. Confocal Microscope C2
 Confocal Microscope Confocal Microscope C2 Confocal Microscope An essential microscopy laboratory insturument The C2 confocal microscope system comprises a new generation of Nikon confocal instruments
Confocal Microscope Confocal Microscope C2 Confocal Microscope An essential microscopy laboratory insturument The C2 confocal microscope system comprises a new generation of Nikon confocal instruments
1 Co Localization and Working flow with the lsm700
 1 Co Localization and Working flow with the lsm700 Samples -1 slide = mousse intestine, Dapi / Ki 67 with Cy3/ BrDU with alexa 488. -1 slide = mousse intestine, Dapi / Ki 67 with Cy3/ no BrDU (but with
1 Co Localization and Working flow with the lsm700 Samples -1 slide = mousse intestine, Dapi / Ki 67 with Cy3/ BrDU with alexa 488. -1 slide = mousse intestine, Dapi / Ki 67 with Cy3/ no BrDU (but with
TRAINING MANUAL. Olympus FV1000
 TRAINING MANUAL Olympus FV1000 September 2014 TABLE OF CONTENTS A. Start-Up Procedure... 1 B. Visual Observation under the Microscope... 1 C. Image Acquisition... 4 A brief Overview of the Settings...
TRAINING MANUAL Olympus FV1000 September 2014 TABLE OF CONTENTS A. Start-Up Procedure... 1 B. Visual Observation under the Microscope... 1 C. Image Acquisition... 4 A brief Overview of the Settings...
Camera Overview. Digital Microscope Cameras for Material Science: Clear Images, Precise Analysis. Digital Cameras for Microscopy
 Digital Cameras for Microscopy Camera Overview For Materials Science Microscopes Digital Microscope Cameras for Material Science: Clear Images, Precise Analysis Passionate about Imaging: Olympus Digital
Digital Cameras for Microscopy Camera Overview For Materials Science Microscopes Digital Microscope Cameras for Material Science: Clear Images, Precise Analysis Passionate about Imaging: Olympus Digital
Camera Overview. Digital Microscope Cameras for Material Science: Clear Images, Precise Analysis. Digital Cameras for Microscopy
 Digital Cameras for Microscopy Camera Overview For Materials Science Microscopes Digital Microscope Cameras for Material Science: Clear Images, Precise Analysis Passionate about Imaging: Olympus Digital
Digital Cameras for Microscopy Camera Overview For Materials Science Microscopes Digital Microscope Cameras for Material Science: Clear Images, Precise Analysis Passionate about Imaging: Olympus Digital
Quick Start Guide. Leica SP5 X
 Quick Start Guide Leica SP5 X Please note: Some of the information in this guide was taken from Leica Microsystems Leica TCS SP5 LAS AF Guide for New Users. This work is licensed under the Creative Commons
Quick Start Guide Leica SP5 X Please note: Some of the information in this guide was taken from Leica Microsystems Leica TCS SP5 LAS AF Guide for New Users. This work is licensed under the Creative Commons
FEMTOSMART. Benefits. Features
 FEMTOSMART Extremely large space under the objective For in vivo studies Field upgradability Patented imaging technologies Flexible scanning methods Maximal photon collection Elevated, column-based body
FEMTOSMART Extremely large space under the objective For in vivo studies Field upgradability Patented imaging technologies Flexible scanning methods Maximal photon collection Elevated, column-based body
LEICA TCS SP5 AOBS TANDEM USER MANUAL
 LEICA TCS SP5 AOBS TANDEM USER MANUAL STARTING THE SYSTEM...2 THE LAS AF SOFTWARE...3 THE «ACQUIRE» MENU...5 CHOOSE AND CREATE A SETTING...6 THE CONTROL PANEL...8 THE DMI6000B MICROSCOPE...10 ACQUIRE ONE
LEICA TCS SP5 AOBS TANDEM USER MANUAL STARTING THE SYSTEM...2 THE LAS AF SOFTWARE...3 THE «ACQUIRE» MENU...5 CHOOSE AND CREATE A SETTING...6 THE CONTROL PANEL...8 THE DMI6000B MICROSCOPE...10 ACQUIRE ONE
Life Science Instrumentation. New Generation. Light Sheet Fluorescence Microscope. Alph
 Life Science Instrumentation Light Sheet Fluorescence Microscope New Generation Alph Modular Light Sheet Microscope Alpha 3 is a new generation of light sheet fluorescence microscope addressing the needs
Life Science Instrumentation Light Sheet Fluorescence Microscope New Generation Alph Modular Light Sheet Microscope Alpha 3 is a new generation of light sheet fluorescence microscope addressing the needs
BASICS OF CONFOCAL IMAGING (PART I)
 BASICS OF CONFOCAL IMAGING (PART I) INTERNAL COURSE 2012 LIGHT MICROSCOPY Lateral resolution Transmission Fluorescence d min 1.22 NA obj NA cond 0 0 rairy 0.61 NAobj Ernst Abbe Lord Rayleigh Depth of field
BASICS OF CONFOCAL IMAGING (PART I) INTERNAL COURSE 2012 LIGHT MICROSCOPY Lateral resolution Transmission Fluorescence d min 1.22 NA obj NA cond 0 0 rairy 0.61 NAobj Ernst Abbe Lord Rayleigh Depth of field
Confocal Microscopy. Kristin Jensen
 Confocal Microscopy Kristin Jensen 17.11.05 References Cell Biological Applications of Confocal Microscopy, Brian Matsumoto, chapter 1 Studying protein dynamics in living cells,, Jennifer Lippincott-Schwartz
Confocal Microscopy Kristin Jensen 17.11.05 References Cell Biological Applications of Confocal Microscopy, Brian Matsumoto, chapter 1 Studying protein dynamics in living cells,, Jennifer Lippincott-Schwartz
Nature Neuroscience: doi: /nn Supplementary Figure 1. Optimized Bessel foci for in vivo volume imaging.
 Supplementary Figure 1 Optimized Bessel foci for in vivo volume imaging. (a) Images taken by scanning Bessel foci of various NAs, lateral and axial FWHMs: (Left panels) in vivo volume images of YFP + neurites
Supplementary Figure 1 Optimized Bessel foci for in vivo volume imaging. (a) Images taken by scanning Bessel foci of various NAs, lateral and axial FWHMs: (Left panels) in vivo volume images of YFP + neurites
The Zeiss AiryScan System, Confocal Four.
 The Zeiss AiryScan System, Confocal Four. Overview. The Zeiss AiryScan module is a segmented, radially stacked GaASP detector and collector system designed to subsample the airy disk of a point emission
The Zeiss AiryScan System, Confocal Four. Overview. The Zeiss AiryScan module is a segmented, radially stacked GaASP detector and collector system designed to subsample the airy disk of a point emission
Basics of confocal imaging (part I)
 Basics of confocal imaging (part I) Swiss Institute of Technology (EPFL) Faculty of Life Sciences Head of BIOIMAGING AND OPTICS BIOP arne.seitz@epfl.ch Lateral resolution BioImaging &Optics Platform Light
Basics of confocal imaging (part I) Swiss Institute of Technology (EPFL) Faculty of Life Sciences Head of BIOIMAGING AND OPTICS BIOP arne.seitz@epfl.ch Lateral resolution BioImaging &Optics Platform Light
Travel to New Dimensions- LSM 880. The Resolution of a Microscope is limited. The Resolution of a Microscope is limited. Image. Image. Object.
 Travel to New Dimensions- LSM 880 LSM 880: The Power of Sensitivity Our Latest Member of the LSM 880 with GaAsP Detectors Sensitivity, and Ease of Use Innovative High-End Laser Scanning Microscopes from
Travel to New Dimensions- LSM 880 LSM 880: The Power of Sensitivity Our Latest Member of the LSM 880 with GaAsP Detectors Sensitivity, and Ease of Use Innovative High-End Laser Scanning Microscopes from
In-Vivo IMAGING SYSTEMS. A complete line of high resolution optical & X-ray systems for pre-clinical imaging
 In-Vivo IMAGING SYSTEMS A complete line of high resolution optical & X-ray systems for pre-clinical imaging In-Vivo Imaging Systems Carestream is a strong, successful, multi-billion dollar, international
In-Vivo IMAGING SYSTEMS A complete line of high resolution optical & X-ray systems for pre-clinical imaging In-Vivo Imaging Systems Carestream is a strong, successful, multi-billion dollar, international
User Guide to the IBIF Leica TCS SP8 MP Confocal Microscope
 User Guide to the IBIF Leica TCS SP8 MP Confocal Microscope This version: 7.24.14. Introduction The IBIF confocal microscope is made available on a fee-for-use-hour basis to all users who have been trained.
User Guide to the IBIF Leica TCS SP8 MP Confocal Microscope This version: 7.24.14. Introduction The IBIF confocal microscope is made available on a fee-for-use-hour basis to all users who have been trained.
Last updated: May 2014 Y.DeGraaf
 FLINDERS MICROSCOPY BIOMEDICAL SERVICES AVAILABLE MICROSCOPES AND SPECIFICATIONS & INFORMATION REGARDING TRAINING FOR NEW USERS Last updated: May 2014 Y.DeGraaf If you have new staff or students (Honours/Masters
FLINDERS MICROSCOPY BIOMEDICAL SERVICES AVAILABLE MICROSCOPES AND SPECIFICATIONS & INFORMATION REGARDING TRAINING FOR NEW USERS Last updated: May 2014 Y.DeGraaf If you have new staff or students (Honours/Masters
Confocal imaging on the Leica TCS SP8. 1) Turn the system on. 2) Use TCS user account. 3) Start LAS X software:
 Confocal imaging on the Leica TCS SP8 1) Turn the system on. 2) Use TCS user account. 3) Start LAS X software: 4) Do not touch the microscope while the software is initializing. Choose your options: Turn
Confocal imaging on the Leica TCS SP8 1) Turn the system on. 2) Use TCS user account. 3) Start LAS X software: 4) Do not touch the microscope while the software is initializing. Choose your options: Turn
Training Guide for Carl Zeiss LSM 880 with AiryScan FAST
 Training Guide for Carl Zeiss LSM 880 with AiryScan FAST ZEN 2.3 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2018) Power ON Routine 1 2 Turn ON Main Switch from the remote control
Training Guide for Carl Zeiss LSM 880 with AiryScan FAST ZEN 2.3 Optical Imaging & Vital Microscopy Core Baylor College of Medicine (2018) Power ON Routine 1 2 Turn ON Main Switch from the remote control
Supplemental Method Information Zeiss LSM710
 Supplemental Method Information Zeiss LSM710 1 Under the Light Path window set up the confocal for imaging a green dye (Alexa488-EGFP). For example, set up the light path as shown here using the 488 nm
Supplemental Method Information Zeiss LSM710 1 Under the Light Path window set up the confocal for imaging a green dye (Alexa488-EGFP). For example, set up the light path as shown here using the 488 nm
Title: Leica SP5 Confocal User Manual
 Title: Leica SP5 Confocal User Manual Date of first issue: 23/10/2015 Date of review: Version: Admin For assistance or to report an issue Office: CG07 or 05 Email: Igmm-imaginghelpdesk@igmm.ed.ac.uk Website:
Title: Leica SP5 Confocal User Manual Date of first issue: 23/10/2015 Date of review: Version: Admin For assistance or to report an issue Office: CG07 or 05 Email: Igmm-imaginghelpdesk@igmm.ed.ac.uk Website:
Shreyash Tandon M.S. III Year
 Shreyash Tandon M.S. III Year 20091015 Confocal microscopy is a powerful tool for generating high-resolution images and 3-D reconstructions of a specimen by using point illumination and a spatial pinhole
Shreyash Tandon M.S. III Year 20091015 Confocal microscopy is a powerful tool for generating high-resolution images and 3-D reconstructions of a specimen by using point illumination and a spatial pinhole
Leica SP8 TCS Users Manual
 Leica SP8 TCS Users Manual Follow the procedure for start up and log on as posted in the lab. Please log on with your account only and do not share your password with anyone. We track and confirm usage
Leica SP8 TCS Users Manual Follow the procedure for start up and log on as posted in the lab. Please log on with your account only and do not share your password with anyone. We track and confirm usage
Systematic Workflow via Intuitive GUI. Easy operation accomplishes your goals faster than ever.
 Systematic Workflow via Intuitive GUI Easy operation accomplishes your goals faster than ever. 16 With the LEXT OLS4100, observation or measurement begins immediately once the sample is placed on the stage.
Systematic Workflow via Intuitive GUI Easy operation accomplishes your goals faster than ever. 16 With the LEXT OLS4100, observation or measurement begins immediately once the sample is placed on the stage.
IN Cell Analyzer 2000
 GE Healthcare IN Cell Analyzer 2000 Cell analysis just got easier Impressively enabling for cell analysis Developed with meticulous attention to the needs of the entire high-content imaging workflow,
GE Healthcare IN Cell Analyzer 2000 Cell analysis just got easier Impressively enabling for cell analysis Developed with meticulous attention to the needs of the entire high-content imaging workflow,
Confocal Laser Scanning Microscopy
 Name of the Core Facility: Confocal Laser Scanning Microscopy CORE Forschungszentrum Immunologie Mainz Welcome to the CSLM Core Facility: The CLSM Core Facility enables working groups to incorporate high
Name of the Core Facility: Confocal Laser Scanning Microscopy CORE Forschungszentrum Immunologie Mainz Welcome to the CSLM Core Facility: The CLSM Core Facility enables working groups to incorporate high
Leica_Dye_Finder :53 Uhr Seite 6 Dye Finder LAS AF
 Dye Finder LAS AF Dye Finder Multicolor live cell fluorescence microscopy is limited by the availability of spectrally separable fluorescent dyes. Fluorescent dyes (or spectral GFP variants) with incongruent
Dye Finder LAS AF Dye Finder Multicolor live cell fluorescence microscopy is limited by the availability of spectrally separable fluorescent dyes. Fluorescent dyes (or spectral GFP variants) with incongruent
Camera Overview. Olympus Digital Cameras for Materials Science Applications: For Clear and Precise Image Analysis. Digital Cameras for Microscopy
 Digital Cameras for Microscopy Camera Overview For Materials Science Microscopes Olympus Digital Cameras for Materials Science Applications: For Clear and Precise Image Analysis Passionate about Imaging
Digital Cameras for Microscopy Camera Overview For Materials Science Microscopes Olympus Digital Cameras for Materials Science Applications: For Clear and Precise Image Analysis Passionate about Imaging
Megapixel FLIM with bh TCSPC Modules
 Megapixel FLIM with bh TCSPC Modules The New SPCM 64-bit Software Abstract: Becker & Hickl have recently introduced version 9.60 of their SPCM TCSPC data acquisition software. SPCM version 9.60 not only
Megapixel FLIM with bh TCSPC Modules The New SPCM 64-bit Software Abstract: Becker & Hickl have recently introduced version 9.60 of their SPCM TCSPC data acquisition software. SPCM version 9.60 not only
Introduction. INSTRUCTION MANUAL CAT XL, 6500-XLCORE, 6500-FL Evos-XL, Evos-XL/Core, Evos-FL
 1 INSTRUCTION MANUAL CAT. 6500-XL, 6500-XLCORE, 6500-FL Evos-XL, Evos-XL/Core, Evos-FL Introduction Experience faster results and easier cell imaging with an EVOS imaging system! An EVOS system is the
1 INSTRUCTION MANUAL CAT. 6500-XL, 6500-XLCORE, 6500-FL Evos-XL, Evos-XL/Core, Evos-FL Introduction Experience faster results and easier cell imaging with an EVOS imaging system! An EVOS system is the
ZEISS LSM 710 CONFOCAL MICROSCOPE USER MANUAL
 ZEISS LSM 710 CONFOCAL MICROSCOPE USER MANUAL START THE SYSTEM... 2 START ZEN SOFTWARE... 3 SET THE TEMPERATURE AND THE CO2 CONTROLLERS... OBSERVATION AT OCULARS... 5 STATIF PRESENTATION... 6 ACQUIRE ONE
ZEISS LSM 710 CONFOCAL MICROSCOPE USER MANUAL START THE SYSTEM... 2 START ZEN SOFTWARE... 3 SET THE TEMPERATURE AND THE CO2 CONTROLLERS... OBSERVATION AT OCULARS... 5 STATIF PRESENTATION... 6 ACQUIRE ONE
Fundamentals of Light Microscopy II: Fluorescence, Deconvolution, Confocal, Multiphoton, Spectral microscopy. Integrated Microscopy Course
 Fundamentals of Light Microscopy II: Fluorescence, Deconvolution, Confocal, Multiphoton, Spectral microscopy Integrated Microscopy Course Review Lecture 1: Microscopy Basics Light train Kohler illumination*
Fundamentals of Light Microscopy II: Fluorescence, Deconvolution, Confocal, Multiphoton, Spectral microscopy Integrated Microscopy Course Review Lecture 1: Microscopy Basics Light train Kohler illumination*
SHORT INSTRUCTIONS FOR OPERATING LSM1/2 (Zeiss LSM510) AT CIAN Version 1.4, September 2014
 CIAN LSM1 or LSM2 short instructions, version 1.4, September 2014 page 1 of 6 SHORT INSTRUCTIONS FOR OPERATING LSM1/2 (Zeiss LSM510) AT CIAN Version 1.4, September 2014 Before starting To work with LSM1
CIAN LSM1 or LSM2 short instructions, version 1.4, September 2014 page 1 of 6 SHORT INSTRUCTIONS FOR OPERATING LSM1/2 (Zeiss LSM510) AT CIAN Version 1.4, September 2014 Before starting To work with LSM1
Quick Guide for Zeiss 710 Laser Scanning Confocal MGH Cancer Center
 Quick Guide for Zeiss 710 Laser Scanning Confocal MGH Cancer Center For any questions or concerns, please contact: Linda Nieman lnieman@mgh.harvard.edu Office: (617) 643-9684 Cell: (512) 565-8076 Chenyue
Quick Guide for Zeiss 710 Laser Scanning Confocal MGH Cancer Center For any questions or concerns, please contact: Linda Nieman lnieman@mgh.harvard.edu Office: (617) 643-9684 Cell: (512) 565-8076 Chenyue
Leica SP8 TCS Users Manual
 Version : 07/08/0 Leica SP8 TCS Users Manual Start up:. Turn the PC Microscope, Scanner Power, Laser Power, and the Laser Emission key to on (bottom right of desk).. Turn on the fluorescent lamp (top left
Version : 07/08/0 Leica SP8 TCS Users Manual Start up:. Turn the PC Microscope, Scanner Power, Laser Power, and the Laser Emission key to on (bottom right of desk).. Turn on the fluorescent lamp (top left
Operation Guide for the Leica SP2 Confocal Microscope Bio-Imaging Facility Hunter College October 2009
 Operation Guide for the Leica SP2 Confocal Microscope Bio-Imaging Facility Hunter College October 2009 Introduction of Fluoresence Confocal Microscopy The first confocal microscope was invented by Princeton
Operation Guide for the Leica SP2 Confocal Microscope Bio-Imaging Facility Hunter College October 2009 Introduction of Fluoresence Confocal Microscopy The first confocal microscope was invented by Princeton
HoloMonitor M4. For powerful discoveries in your incubator
 HoloMonitor M4 For powerful discoveries in your incubator HoloMonitor offers unique imaging capabilities that greatly enhance our understanding of cell behavior, previously unachievable by other technologies
HoloMonitor M4 For powerful discoveries in your incubator HoloMonitor offers unique imaging capabilities that greatly enhance our understanding of cell behavior, previously unachievable by other technologies
Camera Overview. Digital Microscope Cameras for Material Science: Clear Images, Precise Analysis. Digital Cameras for Microscopy
 Digital Cameras for Microscopy Camera Overview For Materials Science Microscopes Digital Microscope Cameras for Material Science: Clear Images, Precise Analysis Passionate about Imaging: Olympus Digital
Digital Cameras for Microscopy Camera Overview For Materials Science Microscopes Digital Microscope Cameras for Material Science: Clear Images, Precise Analysis Passionate about Imaging: Olympus Digital
Point Spread Function. Confocal Laser Scanning Microscopy. Confocal Aperture. Optical aberrations. Alternative Scanning Microscopy
 Bi177 Lecture 5 Adding the Third Dimension Wide-field Imaging Point Spread Function Deconvolution Confocal Laser Scanning Microscopy Confocal Aperture Optical aberrations Alternative Scanning Microscopy
Bi177 Lecture 5 Adding the Third Dimension Wide-field Imaging Point Spread Function Deconvolution Confocal Laser Scanning Microscopy Confocal Aperture Optical aberrations Alternative Scanning Microscopy
Akinori Mitani and Geoff Weiner BGGN 266 Spring 2013 Non-linear optics final report. Introduction and Background
 Akinori Mitani and Geoff Weiner BGGN 266 Spring 2013 Non-linear optics final report Introduction and Background Two-photon microscopy is a type of fluorescence microscopy using two-photon excitation. It
Akinori Mitani and Geoff Weiner BGGN 266 Spring 2013 Non-linear optics final report Introduction and Background Two-photon microscopy is a type of fluorescence microscopy using two-photon excitation. It
Bi/BE 227 Winter Assignment #3. Adding the third dimension: 3D Confocal Imaging
 Bi/BE 227 Winter 2016 Assignment #3 Adding the third dimension: 3D Confocal Imaging Schedule: Jan 20: Assignment Jan 20-Feb 8: Work on assignment Feb 10: Student PowerPoint presentations. Goals for this
Bi/BE 227 Winter 2016 Assignment #3 Adding the third dimension: 3D Confocal Imaging Schedule: Jan 20: Assignment Jan 20-Feb 8: Work on assignment Feb 10: Student PowerPoint presentations. Goals for this
Olympus Fluoview 1000S Spectral Confocal Microscope Introduction to the NRI-MCDB Microscopy Facility Spectral Confocal Microscope
 Olympus Fluoview 1000S Spectral Confocal Microscope Introduction to the NRI-MCDB Microscopy Facility Spectral Confocal Microscope Improved Optics More Lasers 405 diode 440 diode 488 Argon 515 Argon 559
Olympus Fluoview 1000S Spectral Confocal Microscope Introduction to the NRI-MCDB Microscopy Facility Spectral Confocal Microscope Improved Optics More Lasers 405 diode 440 diode 488 Argon 515 Argon 559
ANSWER KEY Lab 2 (IGB): Bright Field and Fluorescence Optical Microscopy and Sectioning
 Phys598BP Spring 2016 University of Illinois at Urbana-Champaign ANSWER KEY Lab 2 (IGB): Bright Field and Fluorescence Optical Microscopy and Sectioning Location: IGB Core Microscopy Facility Microscope:
Phys598BP Spring 2016 University of Illinois at Urbana-Champaign ANSWER KEY Lab 2 (IGB): Bright Field and Fluorescence Optical Microscopy and Sectioning Location: IGB Core Microscopy Facility Microscope:
NIS-Elements C (For CONFOCAL MICROSCOPE A1) Instructions (Ver. 4.40)
 M487E 15.4.NF.17 (1/4) *M487EN17* NIS-Elements C (For CONFOCAL MICROSCOPE A1) Instructions (Ver. 4.40) Preface Thank you for purchasing the Nikon products. This instruction manual has been prepared for
M487E 15.4.NF.17 (1/4) *M487EN17* NIS-Elements C (For CONFOCAL MICROSCOPE A1) Instructions (Ver. 4.40) Preface Thank you for purchasing the Nikon products. This instruction manual has been prepared for
1.The Problem LIGHT-LEVEL LEVEL IMAGING. light-level level Cameras. 3. Solutions. 2. Low-light LOW-LIGHT
 LOW-LIGHT LIGHT-LEVEL LEVEL IMAGING 1.The Problem 2. Low-light light-level level Cameras 3. Solutions How Much Light? I. Illumination system: 75 W Xenon Arc (~1mW/nm in visible) 490/10 nm exciter filter
LOW-LIGHT LIGHT-LEVEL LEVEL IMAGING 1.The Problem 2. Low-light light-level level Cameras 3. Solutions How Much Light? I. Illumination system: 75 W Xenon Arc (~1mW/nm in visible) 490/10 nm exciter filter
Nikon. King s College London. Imaging Centre. N-SIM guide NIKON IMAGING KING S COLLEGE LONDON
 N-SIM guide NIKON IMAGING CENTRE @ KING S COLLEGE LONDON Starting-up / Shut-down The NSIM hardware is calibrated after system warm-up occurs. It is recommended that you turn-on the system for at least
N-SIM guide NIKON IMAGING CENTRE @ KING S COLLEGE LONDON Starting-up / Shut-down The NSIM hardware is calibrated after system warm-up occurs. It is recommended that you turn-on the system for at least
Confocal Microscopy. (Increasing contrast and resolu6on using op6cal sec6oning) Lecture 7. November 2017
 Confocal Microscopy (Increasing contrast and resolu6on using op6cal sec6oning) Lecture 7 November 2017 3 Flavours of Microscope Confocal Laser Scanning Problem: Out of Focus Light Spinning disc 2-Photon
Confocal Microscopy (Increasing contrast and resolu6on using op6cal sec6oning) Lecture 7 November 2017 3 Flavours of Microscope Confocal Laser Scanning Problem: Out of Focus Light Spinning disc 2-Photon
Guide to Confocal 5. Starting session
 Guide to Confocal 5 Remember that when booking and before starting session you can check for any problems at https://www.bris.ac.uk/biochemistry/uobonly/cif/index.html Starting session Switch on microscope
Guide to Confocal 5 Remember that when booking and before starting session you can check for any problems at https://www.bris.ac.uk/biochemistry/uobonly/cif/index.html Starting session Switch on microscope
Modes of light microscopy Choosing the appropriate system
 Modes of light microscopy Choosing the appropriate system Wide-field microscopy Confocal microscopy Multi-photon microscopy Wide-field, inverted fluorescence microscope Nikon MicroscopyU Endosome migration
Modes of light microscopy Choosing the appropriate system Wide-field microscopy Confocal microscopy Multi-photon microscopy Wide-field, inverted fluorescence microscope Nikon MicroscopyU Endosome migration
Rapid Adaptive Optical Recovery of Optimal Resolution over Large Volumes
 SUPPLEMENTARY MATERIAL Rapid Adaptive Optical Recovery of Optimal Resolution over Large Volumes Kai Wang, Dan Milkie, Ankur Saxena, Peter Engerer, Thomas Misgeld, Marianne E. Bronner, Jeff Mumm, and Eric
SUPPLEMENTARY MATERIAL Rapid Adaptive Optical Recovery of Optimal Resolution over Large Volumes Kai Wang, Dan Milkie, Ankur Saxena, Peter Engerer, Thomas Misgeld, Marianne E. Bronner, Jeff Mumm, and Eric
ADVANCED METHODS FOR CONFOCAL MICROSCOPY II. Jean-Yves Chatton Sept. 2006
 ADVANCED METHODS FOR CONFOCAL MICROSCOPY II Jean-Yves Chatton Sept. 2006 Workshop outline Confocal microscopy of living cells and tissues X-Z scanning Time series Bleach: FRAP, photoactivation Emission
ADVANCED METHODS FOR CONFOCAL MICROSCOPY II Jean-Yves Chatton Sept. 2006 Workshop outline Confocal microscopy of living cells and tissues X-Z scanning Time series Bleach: FRAP, photoactivation Emission
SpectraMax i3x Multi-Mode Detection Platform. Explore a wealth of applications in one future-ready system
 SpectraMax i3x Multi-Mode Detection Platform Explore a wealth of applications in one future-ready system Benefits User-upgradeable application modules including cellular imaging Sensitivity across spectrum
SpectraMax i3x Multi-Mode Detection Platform Explore a wealth of applications in one future-ready system Benefits User-upgradeable application modules including cellular imaging Sensitivity across spectrum
Nikon C1si Spectral Laser Scanning Confocal Microscope. User Guide
 Nikon C1si Spectral Laser Scanning Confocal Microscope User Guide Contents: C1Si Turn-On/ShutDown Procedures... 2 Overview... 4 Setup for epi-illumination to view through the eyepieces:... 5 Setup for
Nikon C1si Spectral Laser Scanning Confocal Microscope User Guide Contents: C1Si Turn-On/ShutDown Procedures... 2 Overview... 4 Setup for epi-illumination to view through the eyepieces:... 5 Setup for
Multidimensional Imaging with the Opterra Multipoint Scanning Confocal System
 Multidimensional Imaging with the Opterra Multipoint Scanning Confocal System Outline Opterra design overview Light Path Scan Modes Performance Application data Drosophila larva heart. Courtesy of Nikon
Multidimensional Imaging with the Opterra Multipoint Scanning Confocal System Outline Opterra design overview Light Path Scan Modes Performance Application data Drosophila larva heart. Courtesy of Nikon
Technology Note ZEISS LSM 880 with Airyscan
 Technology Note ZEISS LSM 880 with Airyscan Introducing the Fast Acquisition Mode ZEISS LSM 880 with Airyscan Introducing the Fast Acquisition Mode Author: Dr. Annette Bergter Carl Zeiss Microscopy GmbH,
Technology Note ZEISS LSM 880 with Airyscan Introducing the Fast Acquisition Mode ZEISS LSM 880 with Airyscan Introducing the Fast Acquisition Mode Author: Dr. Annette Bergter Carl Zeiss Microscopy GmbH,
3. are adherent cells (ie. cells in suspension are too far away from the coverslip)
 Before you begin, make sure your sample... 1. is seeded on #1.5 coverglass (thickness = 0.17) 2. is an aqueous solution (ie. fixed samples mounted on a slide will not work - not enough difference in refractive
Before you begin, make sure your sample... 1. is seeded on #1.5 coverglass (thickness = 0.17) 2. is an aqueous solution (ie. fixed samples mounted on a slide will not work - not enough difference in refractive
High-sensitivity. optical molecular imaging and high-resolution digital X-ray. In-Vivo Imaging Systems
 High-sensitivity optical molecular imaging and high-resolution digital X-ray In-Vivo Imaging Systems In vivo imaging solutions available in several packages Carestream Molecular Imaging offers a selection
High-sensitivity optical molecular imaging and high-resolution digital X-ray In-Vivo Imaging Systems In vivo imaging solutions available in several packages Carestream Molecular Imaging offers a selection
Nikon A1R. Multi-Photon & Laser Scanning Confocal Microscope. Kyle Marchuk Adam Fries Jordan Briscoe Kaitlin Corbin. April 2017.
 Nikon A1R Multi-Photon & Laser Scanning Confocal Microscope Kyle Marchuk Adam Fries Jordan Briscoe Kaitlin Corbin April 2017 Contents 1 Introduction 2 2 Start-Up 2 3 Imaging 4 3.1 Sample Alignment...........................................
Nikon A1R Multi-Photon & Laser Scanning Confocal Microscope Kyle Marchuk Adam Fries Jordan Briscoe Kaitlin Corbin April 2017 Contents 1 Introduction 2 2 Start-Up 2 3 Imaging 4 3.1 Sample Alignment...........................................
Nikon SIM-E & A1-R System
 Nikon SIM-E & A1-R System USER GUIDE LSU Health Sciences Center Shreveport Research Core Facility June 01 2017 Chaowei Shang 1 Table of Content 1. Start Up the System... Page 3 Hardware and microscope
Nikon SIM-E & A1-R System USER GUIDE LSU Health Sciences Center Shreveport Research Core Facility June 01 2017 Chaowei Shang 1 Table of Content 1. Start Up the System... Page 3 Hardware and microscope
Nikon A1Rsi Confocal Start-Up Sequence
 1. Turn the key on the Nikon LUN-V Laser Launch. Nikon A1Rsi Confocal Start-Up Sequence 2. Press the button the left side of the A1Rsi Controller unit. 3. Turn on the power strip underneath the microscope.
1. Turn the key on the Nikon LUN-V Laser Launch. Nikon A1Rsi Confocal Start-Up Sequence 2. Press the button the left side of the A1Rsi Controller unit. 3. Turn on the power strip underneath the microscope.
Pixel shift in fluorescence microscopy
 Pixel shift in fluorescence microscopy 1. Introduction Multicolor imaging in fluorescence microscopy is typically performed by sequentially acquiring images of different colors. An overlay of these images
Pixel shift in fluorescence microscopy 1. Introduction Multicolor imaging in fluorescence microscopy is typically performed by sequentially acquiring images of different colors. An overlay of these images
HoloMonitor. Phase. For competent and powerful discoveries. Holographic time-lapse imaging cytometry
 HoloMonitor M4 Holographic time-lapse imaging cytometry For competent and powerful discoveries Monitor and quantify living cells in their natural environment with unrivaled temporal resolution Phase Holographic
HoloMonitor M4 Holographic time-lapse imaging cytometry For competent and powerful discoveries Monitor and quantify living cells in their natural environment with unrivaled temporal resolution Phase Holographic
Fastest high definition Raman imaging. Fastest Laser Raman Microscope RAMAN
 Fastest high definition Raman imaging Fastest Laser Raman Microscope RAMAN - 11 www.nanophoton.jp Observation A New Generation in Raman Observation RAMAN-11 developed by Nanophoton was newly created by
Fastest high definition Raman imaging Fastest Laser Raman Microscope RAMAN - 11 www.nanophoton.jp Observation A New Generation in Raman Observation RAMAN-11 developed by Nanophoton was newly created by
Leica Sp5 II Confocal User Guide
 Leica Sp5 II Confocal User Guide Turning on the Confocal System (instructions are posted in the room) 1. Turn on Laser Power Button 2. Turn Key to On position 3. Turn on Scanner Power Button 4. Turn on
Leica Sp5 II Confocal User Guide Turning on the Confocal System (instructions are posted in the room) 1. Turn on Laser Power Button 2. Turn Key to On position 3. Turn on Scanner Power Button 4. Turn on
Chemical Imaging. Whiskbroom Imaging. Staring Imaging. Pushbroom Imaging. Whiskbroom. Staring. Pushbroom
 Chemical Imaging Whiskbroom Chemical Imaging (CI) combines different technologies like optical microscopy, digital imaging and molecular spectroscopy in combination with multivariate data analysis methods.
Chemical Imaging Whiskbroom Chemical Imaging (CI) combines different technologies like optical microscopy, digital imaging and molecular spectroscopy in combination with multivariate data analysis methods.
FV1200 FLUOVIEW. High-Performance Laser Scanning Microscope for Live Cell Imaging Combining Accuracy, Sensitivity and Laser Stimulation
 Images are courtesy of the following institutions: Dopaminergic neural circuits of the fruit fly Drosophila brain (adult female). Three-channel antibody labeling of the brain in which an expression driver
Images are courtesy of the following institutions: Dopaminergic neural circuits of the fruit fly Drosophila brain (adult female). Three-channel antibody labeling of the brain in which an expression driver
長庚大學共軛焦顯微鏡課程 長庚大學共軛焦顯微鏡課程. Spot light 長庚大學
 長庚大學共軛焦顯微鏡課程 Spot light 長庚大學共軛焦顯微鏡課程 20071030 長庚大學 Basic principle of Laser Scanning Confocal Microscopy The application of LSM 510 META detector Multiphoton microscopy basic principle and introduction
長庚大學共軛焦顯微鏡課程 Spot light 長庚大學共軛焦顯微鏡課程 20071030 長庚大學 Basic principle of Laser Scanning Confocal Microscopy The application of LSM 510 META detector Multiphoton microscopy basic principle and introduction
